19. Reactions of Aromatics: EAS and Beyond
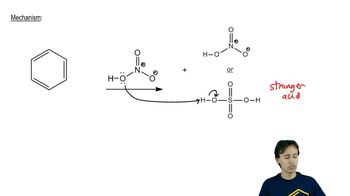
EAS:Nitration Mechanism
Practice this topic
- Multiple ChoicePredict the major, organic product for the following reaction.259views
- Textbook Question
Propose a mechanism for nitration of pyridine at the 4-position,
and show why this orientation is not observed.
275views - Textbook Question
Before spectroscopy was invented, Körner’s absolute method was used to determine whether a disubstituted benzene derivative was the ortho, meta, or para isomer. Körner’s method involves adding a third group (often a nitro group) and determining how many isomers are formed. For example, when o-xylene is nitrated (by a method shown in Chapter 17), two isomers are formed.
(a) How many isomers are formed by nitration of m-xylene?
243views - Textbook Question
Before spectroscopy was invented, Körner’s absolute method was used to determine whether a disubstituted benzene derivative was the ortho, meta, or para isomer. Körner’s method involves adding a third group (often a nitro group) and determining how many isomers are formed. For example, when o-xylene is nitrated (by a method shown in Chapter 17), two isomers are formed.
(b) How many isomers are formed by nitration of p-xylene?
286views - Textbook Question
Before spectroscopy was invented, Körner’s absolute method was used to determine whether a disubstituted benzene derivative was the ortho, meta, or para isomer. Körner’s method involves adding a third group (often a nitro group) and determining how many isomers are formed. For example, when o-xylene is nitrated (by a method shown in Chapter 17), two isomers are formed.
(c) A turn-of-the-century chemist isolated an aromatic compound of molecular formula C6H4Br2. He carefully nitrated this compound and purified three isomers of formula C6H3Br2NO2. Propose structures for the original compound and the three nitrated derivatives
208views