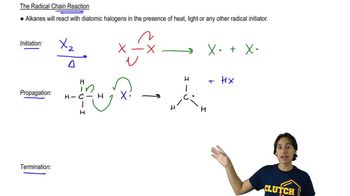
11. Radical Reactions
Free Radical Halogenation
11. Radical Reactions
Free Radical Halogenation
Practice this topic
- Multiple ChoicePredict the major, organic product of the following reaction.423views
- Multiple ChoiceWhich step of a radical reaction is responsible for the formation of the bulk of the product?354views
- Textbook QuestionFree-radical chlorination of hexane gives very poor yields of 1-chlorohexane, while cyclohexane can be converted to chlorocyclohexane in good yield. a. How do you account for this difference? b. What ratio of reactants (cyclohexane and chlorine) would you use for the synthesis of chlorocyclohexane?1332views
- Textbook Questionc. How could an industrial plant control the proportions of methane and chlorine to favor production of CCl4? To favor production of CH3Cl?604views
- Textbook QuestionPeroxides are often added to free-radical reactions as initiators because the oxygen–oxygen bond cleaves homolytically rather easily. For example, the bond-dissociation enthalpy of the O―O bond in hydrogen peroxide (H―O―O―H) is only 213 kJ/mol (51 kcal/mol). Give a mechanism for the hydrogen peroxide-initiated reaction of cyclopentane with chlorine. The BDE for HO―Cl is 210 kJ/mol (50 kcal/mol).1921views1rank
- Textbook QuestionWhen exactly 1 mole of methane is mixed with exactly 1 mole of chlorine and light is shone on the mixture, a chlorination reaction occurs. The products are found to contain substantial amounts of di-, tri-, and tetrachloromethane, as well as unreacted methane. a. Explain how a mixture is formed from this stoichiometric mixture of reactants, and propose mechanisms for the formation of these compounds from chloromethane.874views