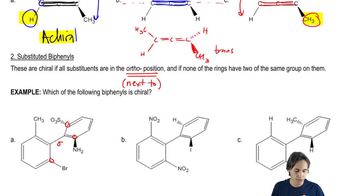
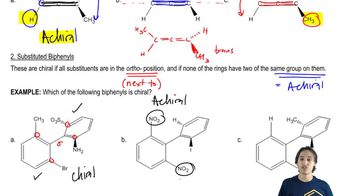
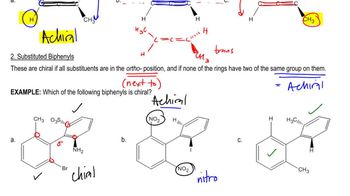
5. Chirality
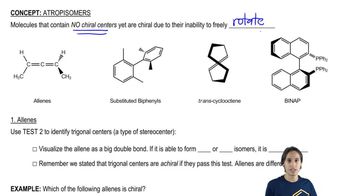
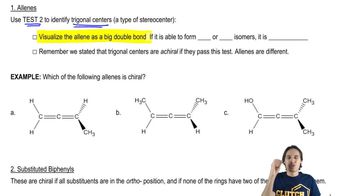
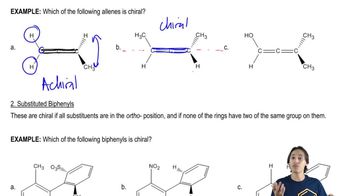
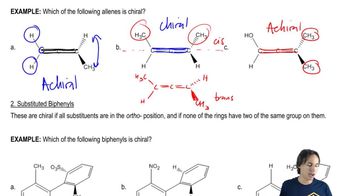
Atropisomers
5. Chirality
Atropisomers
Showing 7 of 7 videos
Practice this topic
- Textbook QuestionThe original definition of meso is 'an achiral compound that has chiral diastereomers.' Our working definition of meso is 'an achiral compound that has chiral centers (usually asymmetric carbon atoms).' The working definition is much easier to apply, because we don't have to envision all possible chiral diastereomers of the compound. Still, the working definition is not quite as complete as the original definition. 1. Show how cis-cyclooctene is defined as a meso compound under the original definition, but not under our working definition. [Review FIGURE 5-19 ]1136views
- Textbook Question
Draw three-dimensional representations of the following compounds. Which have asymmetric carbon atoms? Which have no asymmetric carbons but are chiral anyway? Use your models for parts (a) through (d) and any others that seem unclear.
(g)
710views - Textbook Question(••••) A compound with two chiral centers that is meso will always have opposite absolute configurations at the two chiral centers. That is, a meso compound will never be (R,R) or (S,S); instead, it will be (R,S). Explain why this must be true.726views
- Textbook QuestionWhich of the following compounds has a stereoisomer that is a meso compound? C 2,4-dimethylpentane D 1,3-dichlorocyclohexane773views