15. Analytical Techniques:IR, NMR, Mass Spect
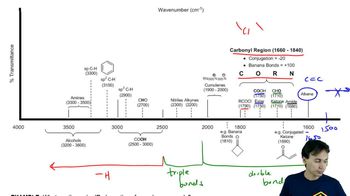
Infrared Spectroscopy Table
15. Analytical Techniques:IR, NMR, Mass Spect
Infrared Spectroscopy Table
Showing 5 of 5 videos
Practice this topic
- Open Question
Answer each of the following questions based on the images below.
a) Which compounds show an intense peak ~ 1700 cm-1?
b) Which compound shows an intense, broad peak at ~ 3400 cm-1?
c) Which compound has a peak at ~1700 cm-1, but no peaks at 2700 cm-1?
d) Which compound has no signal beyond the fingerprint region?
785views12rank3comments - Multiple Choice
Identify which carbonyl group will exhibit a signal at a lower wavenumber
888views9rank1comments - Multiple ChoiceWhich molecule would you expect tohave the lowest wavenumber for the C=O π bond IR band?354views
- Multiple ChoiceAn IR spectrum has bands at ~ 2100 cm−1 and ~ 3300 cm−1. What functional group does this molecule likely represent?564views
- Textbook QuestionThe infrared spectra for three compounds are provided. Each compound has one or more of the following functional groups: conjugated ketone, ester, amide, nitrile, and alkyne. Determine the functional group(s) in each compound, and assign the major peaks above 1600 cm-1. < of IR spectra>658views
- Textbook QuestionSpectra are given for three compounds. Each compound has one or more of the following functional groups: alcohol, amine, ketone, aldehyde, and carboxylic acid. Determine the functional group(s) in each compound, and assign the major peaks above 1600 cm-1. < of IR spectra>549views
- Textbook QuestionA common lab experiment is the dehydration of cyclohexanol to cyclohexene. (a) Explain how you could tell from the IR spectrum whether your product was pure cyclohexene, pure cyclohexanol, or a mixture of cyclohexene and cyclohexanol. Give approximate frequencies for distinctive peaks. (b) Explain why mass spectrometry might not be a good way to distinguish cyclohexene from cyclohexanol.904views