12. Alcohols, Ethers, Epoxides and Thiols
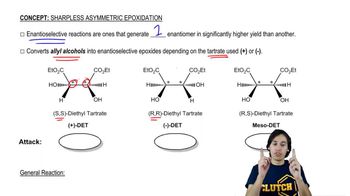
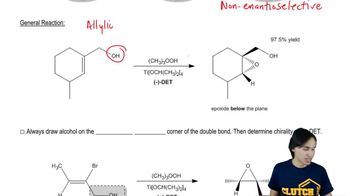
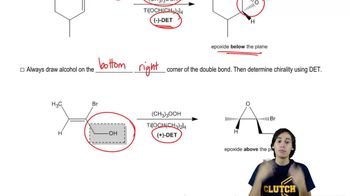
Sharpless Epoxidation
12. Alcohols, Ethers, Epoxides and Thiols
Sharpless Epoxidation
Practice this topic
- Textbook QuestionThe 2001 Nobel Prize in Chemistry was awarded to three organic chemists who have developed methods for catalytic asymmetric syntheses. An asymmetric (or enantioselective) synthesis is one that converts an achiral starting material into mostly one enantiomer of a chiral product. K. Barry Sharpless (The Scripps Research Institute) developed an asymmetric epoxidation of allylic alcohols that gives excellent chemical yields and greater than 90% enantiomeric excess. The Sharpless epoxidation uses tert-butyl hydroperoxide, titanium(IV) isopropoxide, and a dialkyl tartrate ester as the reagents. The following epoxidation of geraniol is typical. < of reactions> (a) Which of these reagents is most likely to be the actual oxidizing agent? That is, which reagent is reduced in the reaction? What is the likely function of the other reagents? (b) When achiral reagents react to give a chiral product, that product is normally formed as a racemic mixture of enantiomers. How can the Sharpless epoxidation give just one nearly pure enantiomer of the product? (c) Draw the other enantiomer of the product. What reagents would you use if you wanted to epoxidize geraniol to give this other enantiomer?559views
- Textbook Question
Identify the alkene that would react with Ti(OiPr)₄, (+) -diethyltartrate, and t-butylhydroperoxide to give the following chiral, nonracemic epoxides.
(a) <IMAGE>
295views - Textbook Question
Identify the alkene that would react with Ti(OiPr)₄, (+) -diethyltartrate, and t-butylhydroperoxide to give the following chiral, nonracemic epoxides.
(b) <IMAGE>
264views - Textbook Question
Identify the alkene that would react with Ti(OiPr)₄, (+) -diethyltartrate, and t-butylhydroperoxide to give the following chiral, nonracemic epoxides.
(c) <IMAGE>
250views