- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

Compound X can be oxidized by chromic acid to form a benzenedicarboxylic acid. Draw the structure of compound X from the given 1H NMR spectrum.

What is the structure of the compound that has the following 1H NMR spectrum data and molecular formula of C5H11Cl?
1 H quintet at 3.38 ppm, 1 H octet at 1.91 ppm, 3 H doublet at 1.55 ppm, 6 H doublet at 0.88 ppm
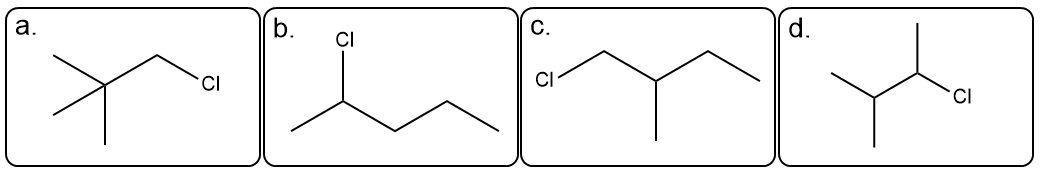
True or False. The 1H NMR spectrum below corresponds to the given compound.
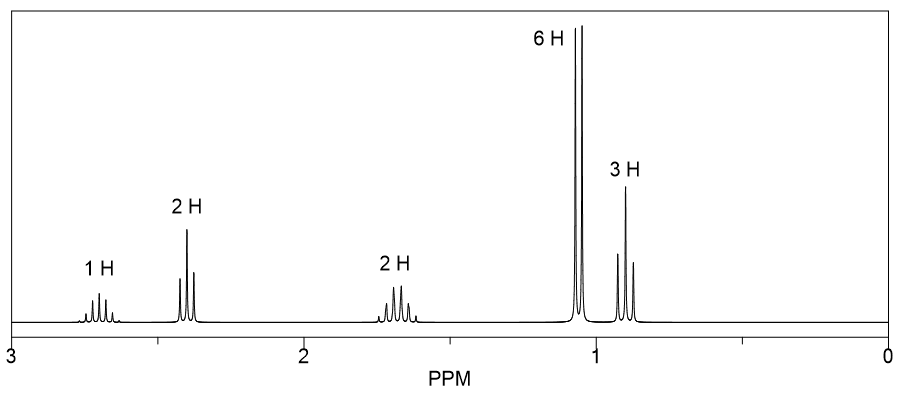

Which of the three isomers with the molecular formula C6H12O has the following 1H NMR spectrum?
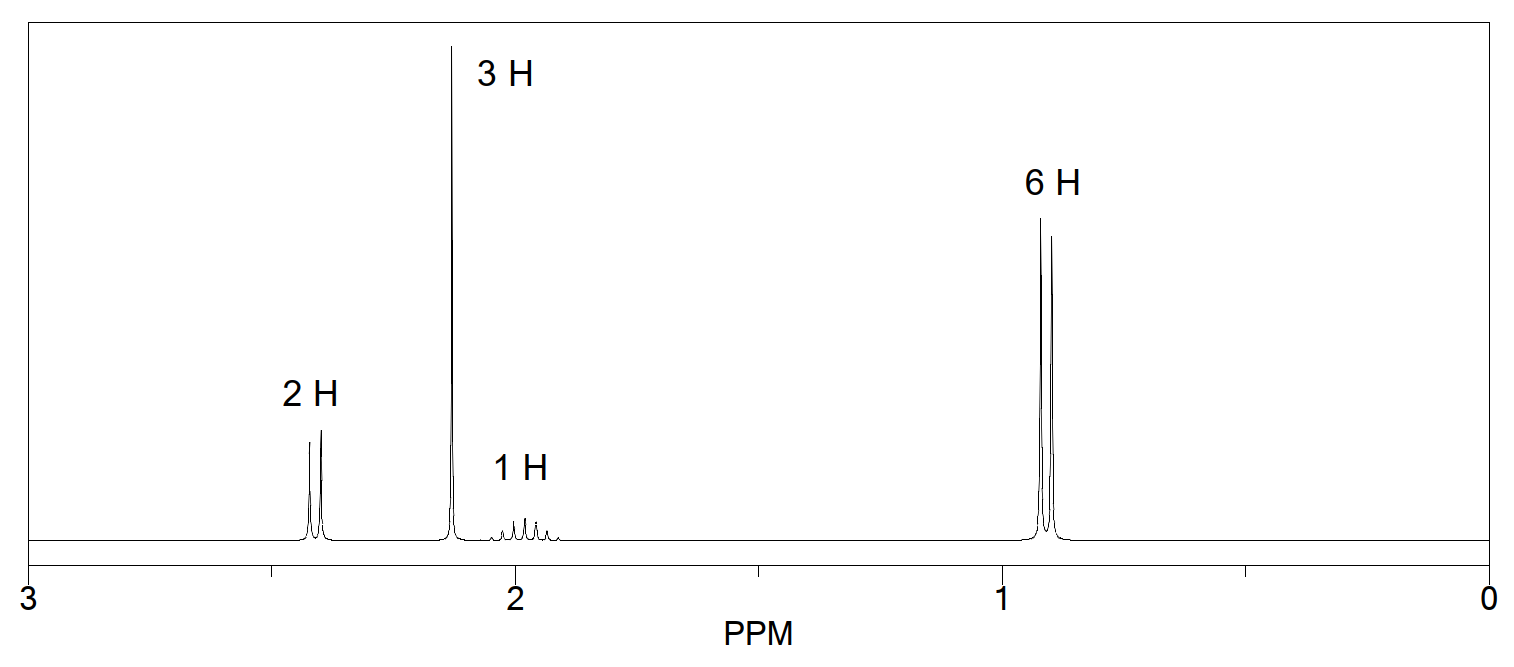
Propose a structure of the compound with molecular formula C5H12O that gives the following NMR spectrum. Assign the molecule's protons, giving rise to labeled peaks in the spectrum.
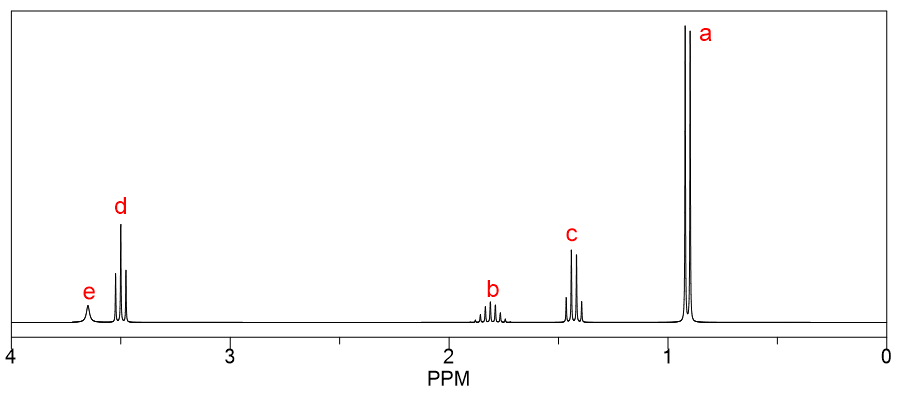
Determine the structure for the following NMR spectrum and molecular formula. Also, state the type of protons that give the peaks at 2.5 ppm and 2.7 ppm.
Molecular Formula: C6H10O3
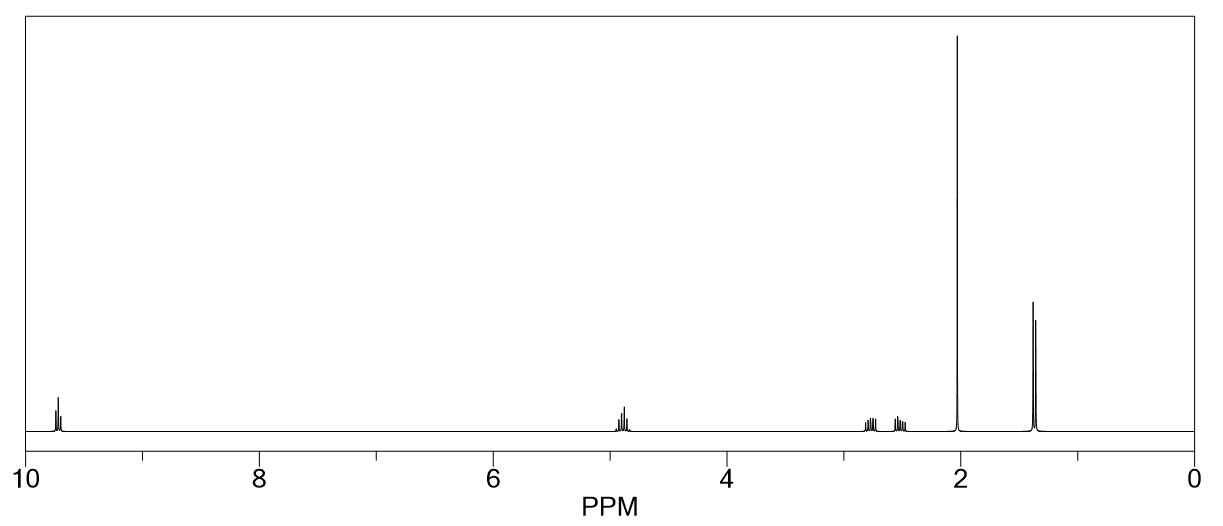
Identify the NMR spectrum for ethyl isobutyrate from the options below and identify the two main peaks that make it different from the spectrum of isopropyl propionate.