- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

Sort 1-propoxypropane (CH3CH2CH2OCH2CH2CH3), water (H2O), and propan-1-ol (CH3CH2CH2OH) in increasing order of their ability to dissolve the following:

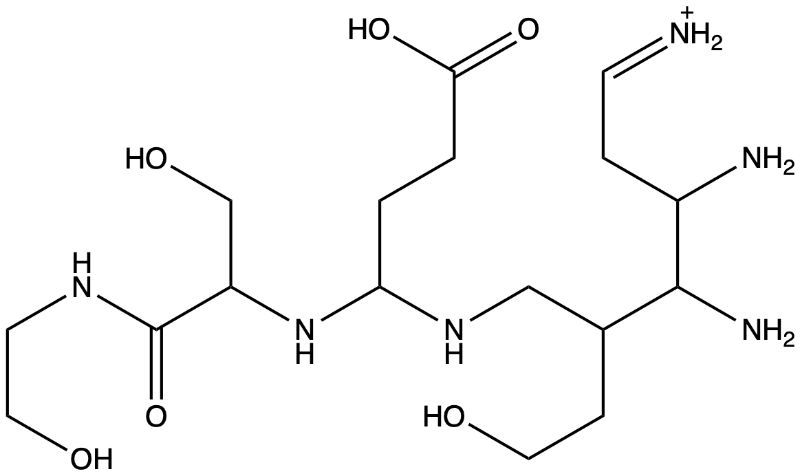
Give two labels for the given structure (choose from hydrophilic, hydrophobic, lipophilic, or lipophobic):
Determine which compound is more soluble in water for each of the following pairs (i): CH3OCH3 or CH3CH2CH3, (ii): CH3OCH3 or CH3CH2OH, (iii): CH3NHCH3 or CH3CH2CH3, (iv): CH3OH or CH3CH2CH2OH, (v): butanone or cycloheptanone.

Among which of the following solvents cyclopentane is the least soluble?
- Water, benzene, chloroform, and 1-butanol
Which of the following pair of alcohols is more soluble in water? Explain your answer.

Amino acids like most amines and carboxylic acids are generally soluble in nonpolar solvents. Is this statement true or false? Justify your answer.
Lorazepam and diazepam are benzodiazepine medications used to treat anxiety disorders, seizures, and alcohol withdrawal symptoms. Diazepam is more lipid-soluble than lorazepam, making lorazepam the recommended drug for patients with hepatic impairment to reduce the risk of accumulation of these drugs in fatty tissues. Explain why lorazepam is less lipid-soluble than diazepam.