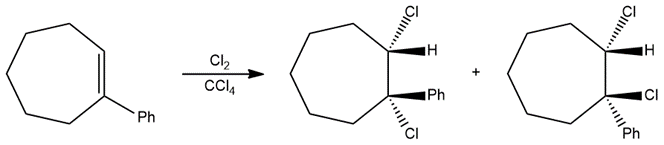
Halogenation reactions of alkenes proceed via the halonium ion mechanism and form the anti-addition product. However, a mixture of the cis and trans isomers formed in the reaction shown below. Propose a reaction mechanism for the formation of the products and explain why the reaction is not stereospecific.