- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

The equilibrium constant (Keq) of a reaction can be calculated using the following equation.
- Keq = e−∆E/RT
Calculate the equilibrium constant for the following reaction at room temperature (298K).

A. Keq = 6.0
B. Keq = 0.006
C. Keq = 6000
D. Keq = 6.066
Consider the following equilibrium process and its related ∆G°:

(1) Calculate Keq.
(2) Determine the % reactants and % products present in an equilibrium mixture at 298 K.
If the temperature is increased, which direction of the reaction, if any, would be favored?

Determine if the equilibrium constant is going to be greater than, equal to, or less than 1 for the following equilibrium reaction. Justify your answer.

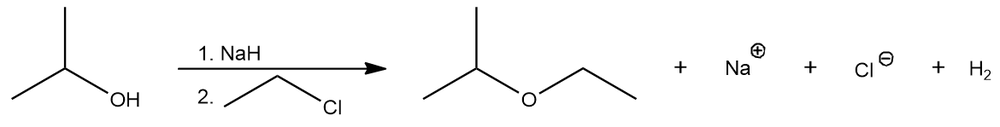
Consider the following reaction:
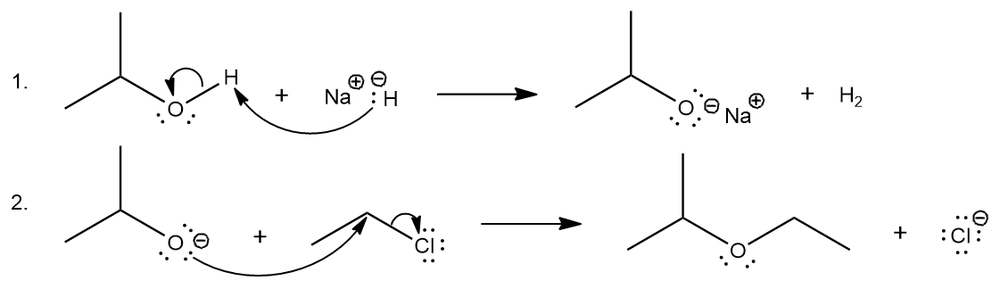
This reaction occurs through the following mechanism:
Explain why the second step of the reaction mechanism is favored and estimate the value of Keq based on the stability of the anions.
If reactant X and product Y are in equilibrium, determine the relative amounts of X and Y present at 30°C when (i) ∆G° = 4.76 kcal and (ii) ∆G° = 0.78 kcal.
Which properties are affected when a reaction is performed with a catalyst compared to the same reaction without a catalyst?
∆G°, ∆H‡, Ea, ∆S‡, ∆H°, Keq, ∆G‡, ∆S°, k