Gibbs Free Energy
6. Thermodynamics and Kinetics / Gibbs Free Energy / Problem 5
Consider the following reaction:
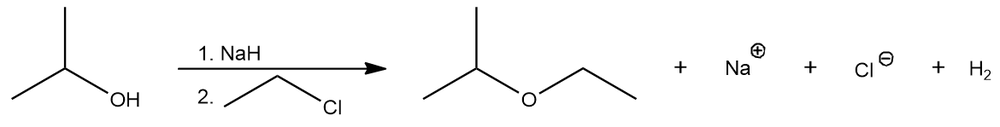
This reaction occurs through the following mechanism:
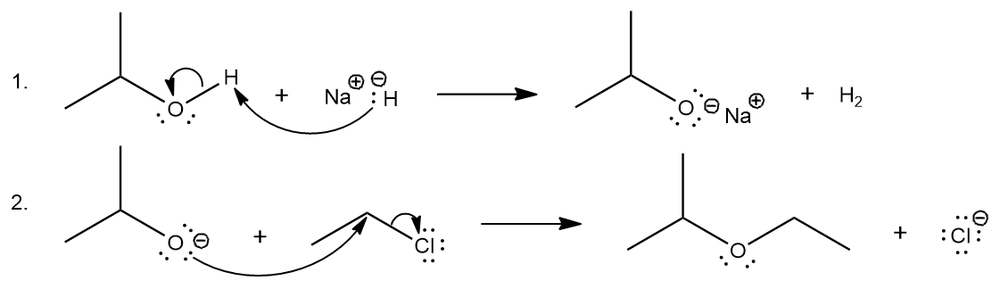
Explain why the second step of the reaction mechanism is favored and estimate the value of Keq based on the stability of the anions.

Learn this concept