- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

Which of the given solvents will be compatible with the acids and bases involved in the following reactions (Ignoring any side reactions)?
Solvent choices = diethyl ether, ethanol, and water.
a. CH3CH2Li + CH3—C≡C—H → CH3—CH3 + CH3—C≡CLi
b. CH3CH2Li + (CH3)2CH—OH → CH3—CH3 + (CH3)2CH—OLi
Using the given Keq value, identify the weakest acid and the weakest base in the reaction given below.

Which of the compounds given below can be deprotonated by CH3O− in an acid-base reaction that favors product formation? (pKa of CH3OH = 15.9)
(i) HCOOH (pKa = 3.7)
(ii) CH3NH2 (pKa = 40)
(iii) NH4+ (pKa = 9.4)
(iv) HC≡CH (pKa = 25)
Ethoxide cannot be used to deprotonate cyclohexane in a reaction that favors the formation of a carbanion. Explain why this is true.
An acid-base equilibrium favors the formation of the weaker acid. Which of the following reactions favor the formation of products?

Some equations of acid-base reactions are given below. Write the products in each case if any significant reaction is possible.
a. CH3—C≡C—H + NaOH
b. CH3—C≡C—H + CH3Li
c. CH3—C≡C—H + NaNH2
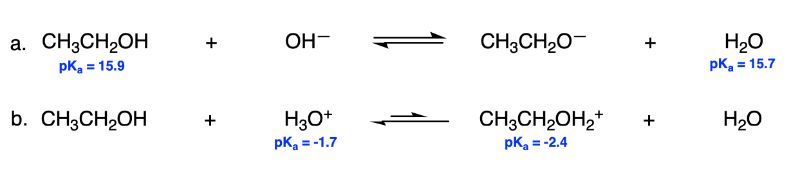
For the acid-base reactions below, prove that the equilibrium lies in the direction indicated in the reactions by comparing the pKa values of the acids on both sides of the equilibrium arrow.