6. Thermodynamics and Kinetics - Part 1 of 2
6. Thermodynamics and Kinetics / Enthalpy / Problem 9
Refer to the information given in the table below. Provide the heat of hydrogenation (∆H°hydr) for each step involved in the reduction of allylbenzene.
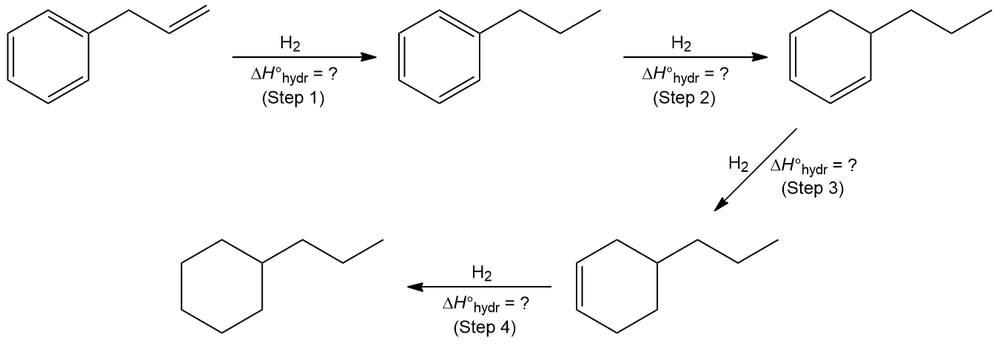
∆H°hydr (Step 1) + ∆H°hydr (Step 2) + ∆H°hydr (Step 3) + ∆H°hydr (Step 4) = ∆H°tot = –78.2 kcal/mol (–328 kJ/mol)


Learn this concept