- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

Identify whether each of the following pairs represents constitutional isomers, enantiomers, diastereomers, or identical compounds.

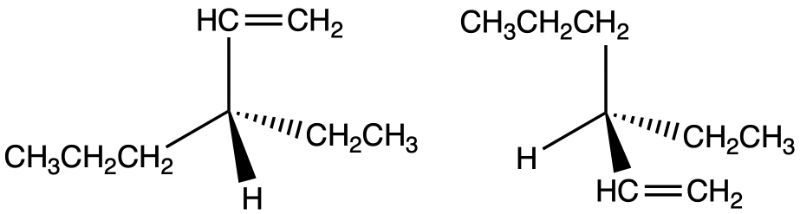
For the given structures of a molecule below, determine if they are identical or a pair of enantiomers.
Transform the Fischer projections below into bond-line structure formulas:

Transform the structural formulas below into Fischer projection formulas:

Transform the following line-angle representation into its corresponding Fischer projection.

Draw a Fischer projection for each of the following compounds and tag any asymmetric carbon with an asterisk (*). Keep the carbon chain vertical with carbon 1 at the top.
a. (R)-Butane-1,2-diol
b. (S)-2-Bromobutane
Determine the configuration (R or S) of the bottom chiral carbon in D-glucose and L-glucose
Assign the absolute configurations to the chiral centers in the following structures.

Among the following structures, identify the optically active stereoisomers.

(S)-1-Bromo-2-methylpentane reacts with the hydroxide ion to produce 2-methyl-1-pentanol without any change in spatial arrangement around the stereocenter. The resulting alcohol rotates the plane-polarized light clockwise. Determine the absolute configuration of (−)-2-methyl-1-pentanol.

A compound has a molecular formula of C2H2Br2Cl2. Draw the possible structures of the compound if it is optically active.
Limonene is a volatile liquid found in citrus fruits. The (R)-enantiomer is used as a flavoring agent in foods and fragrances in the cosmetic industry. Pure (R)-Limonene has a specific rotation [α]D = +115°. Predict the specific rotation of a mixture that contains 82.0 % (R) and 18.0 % (S).

A substance has a specific rotation of −40.00°. In a polarimeter tube that is 5.000 cm long, a solution of the compound (0.8100 g/100.0 mL) has an observed rotation of −7.520°. How much of each enantiomer is in the solution?
An unknown drug has a specific rotation of +82°. The commercial synthesis of this drug results in a mixture with an enantiomeric excess of 94 %.
i. Determine the absolute configuration of this drug.
ii. What is the percentage of each enantiomer in the commercial product?
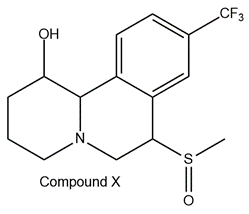
Refer to the structure of Compound X below. How many asymmetric centers does it contain?