- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

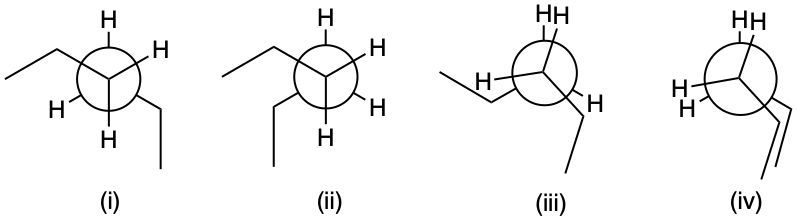
Draw a potential energy diagram for the conformational analysis of n-butane as it rotates around the single bond between C2 and C3.
Hint: One C—H bond eclipsed with another C—H bond contributes to 4.2 KJ/mol, one C—H bond eclipsed with a C—CH3 bond contributes 5.4 KJ/mol while One C—CH3 bond eclipsed with a C—CH3 bond contributes 13 KJ/mol to torsional energy. Similarly, Gauche (60° methyl group) will add 3.8 KJ/mol energy.
Which conformation is the least stable and most stable?
Use the appropriate Newman projection for 2-methylbutane about the C2-C3 bond to write the most stable conformer for 2-methylbutane.

Two conformers can be drawn for the trans isomer of 1,2-dimethylcyclohexane.
(i) Draw Newman projections of the two conformers.
(ii) Which conformer is present in greatest concentration at equilibrium?
There are two possible conformers for cis-1,3-difluorocyclohexane.
(i) Using Newman projections, draw each conformer.
(ii) Identify the conformer that will predominantly exist at equilibrium.
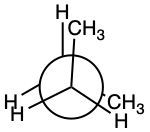
Consider the following:
What is the value for the total strain of the eclipsed conformation?
Assume that the temperature is 298 K. Consider the following bond rotation and use the given table to answer the questions below:


(a) Which conformation is more stable?
(b) Determine the ΔG° for the process.
(c) Determine the equilibrium constant (Keq).
(d) Provide the transition state for the process.
cis-1,2-diethylcyclopropane has a larger heat of combustion than that of the trans isomer. Determine the more stable isomer. Explain your answer using drawings.
The normal bond angle for an sp3-hybridized carbon is 109.5°. The normal C(sp3)-C(sp3) bond length is 1.54 Å. Provide a brief explanation as to why the molecule below deviates from the normal values.

Draw the appropriate structure of 1,2,3,4,5,6-hexafluorocyclohexane with:
(i) all axial positions occupied by F atoms.
(ii) all equatorial positions occupied by F atoms.
Draw the appropriate chair conformers for every stereoisomer of the following compound.
trans-1-chloro-3-ethylcyclohexane
Indicate whether the substituents in the two chair conformers for the disubstituted cyclohexanes given below are one axial and one equatorial in each of the chair conformers or both axial in one chair conformer and both equatorial in the other conformer.
(i) cis-1,3-diethylcyclohexane
(ii) trans-1,3-diethylcyclohexane
Refer to the representations below to complete the table. Fill out the table with the required positions (axial = a; equatorial = e) of the two groups for a conformation to be cis or trans. For example, two groups that must be both equatorial should be denoted as (e,e).