- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

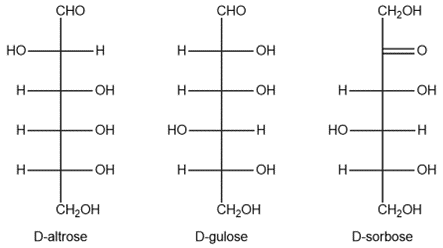
Provide the structure and the name of the products formed in the bromine water oxidation of
(i) D-altrose
(ii) D-gulose
(iii) D-sorbose
Write the name of the following monosaccharide and determine whether it is a reducing or a non-reducing sugar.

Determine whether the molecules below are reducing sugars or not.
(i) α-D-galactopyranose
(ii) methyl β-D-xylofuranoside
Which of the following pairs of aldohexoses produces identical aldaric acids when oxidized?
Determine the number of aldaric acids produced from the eight D-aldohexoses.
When a modified Kiliani-Fischer synthesis begins with L-lyxose, what monosaccharides are produced?
Determine the monosaccharides produced by the modified Kiliani-Fischer synthesis when L-erythrose is used as the starting material.
Provide a suitable mechanism to show the elimination of the cyano group (last step) in the following Wohl degradation reaction.

Identify the two monosaccharides that will form D-xylose via Wohl degradation.
Show that the Ruff degradation of either D-allose or D-altrose results in the formation of the same product, D-ribose.
Ruff degradation of D-talose produces the same product as the Ruff degradation of D-galactose. D-galactose is an aldohexose and C4 epimer of glucose. What is the structure of D-talose?
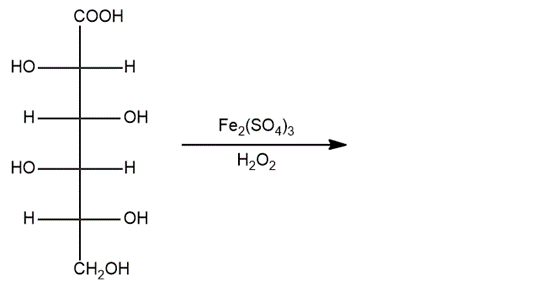
Show the product that will be obtained from the reaction shown below: