- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

Write the structure: (i) the C2 epimer of D-glucose, (ii) the C3 epimer of D-galactose.
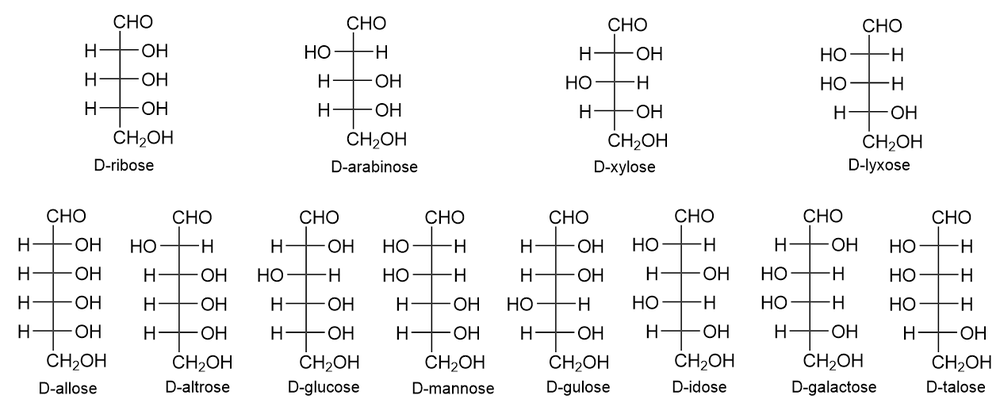
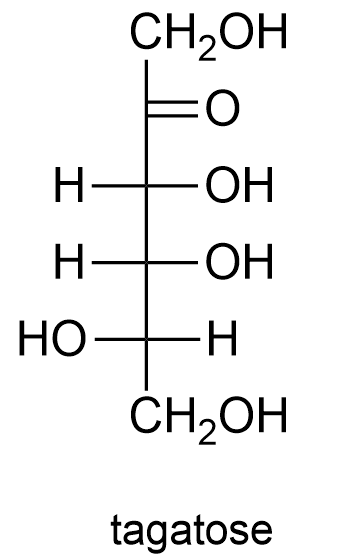
Use the following figures to name the given monosaccharides.
(a) C2 epimer of D-allose
(b) C4 epimer of D-galactose
(c) C3 epimer of D-xylose
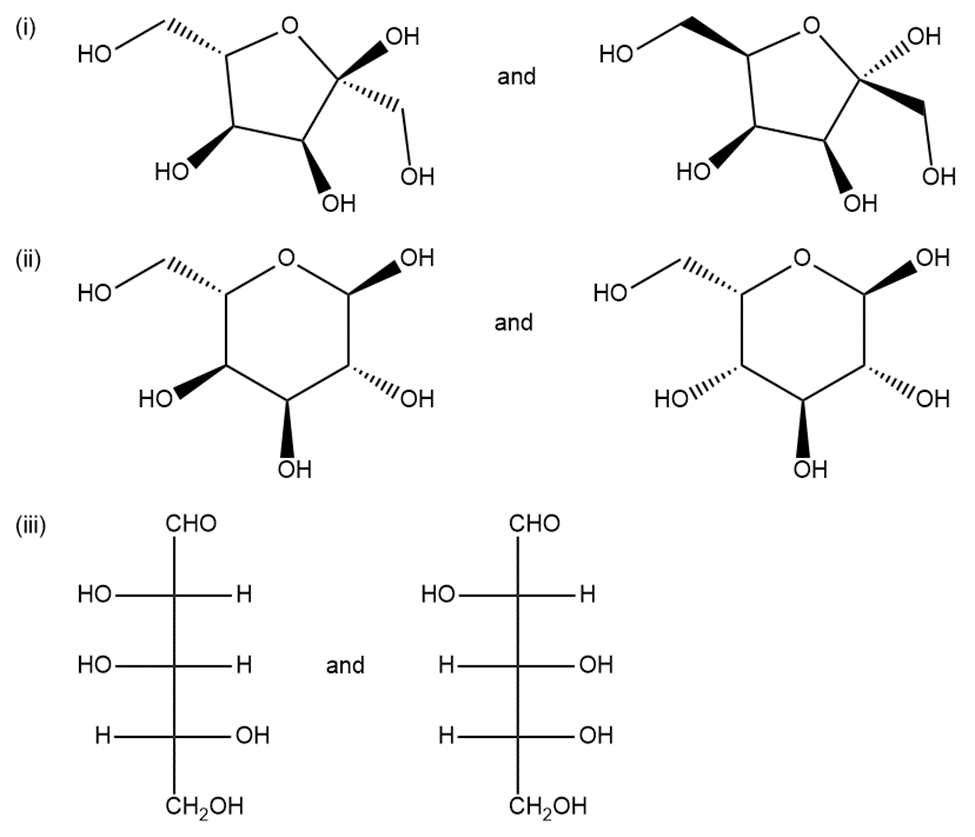
Which pair of molecules below can be classified as epimers?
Is the following statement correct or incorrect?
People with type O blood can receive blood from anyone, but they cannot donate blood to everyone because this blood type lacks a specific sugar component.
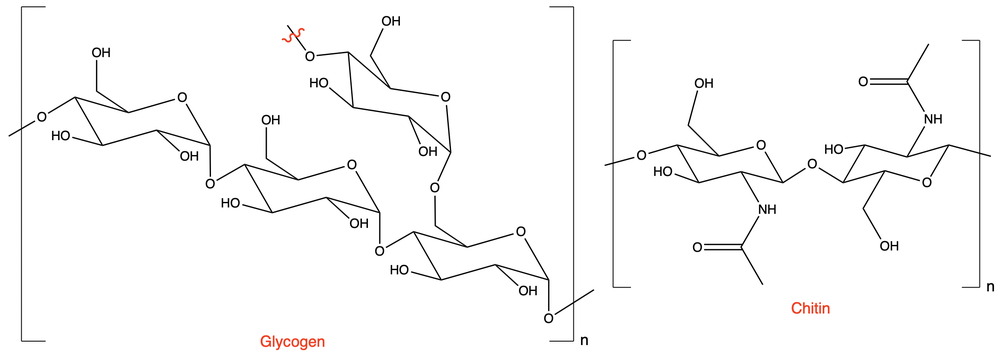
What are the structural differences between the polysaccharides: glycogen and chitin?
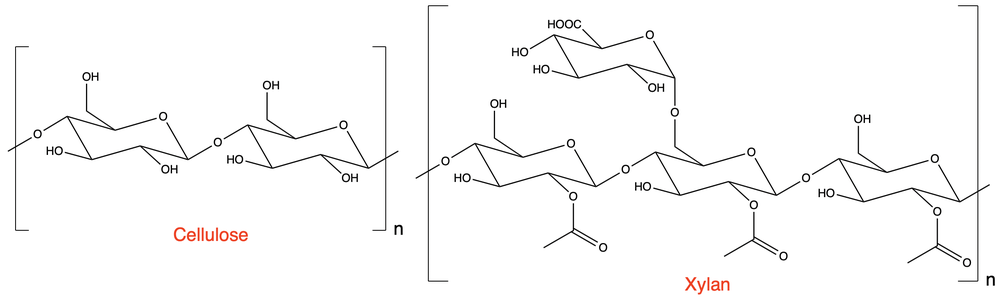
For the given polysaccharides:
What are the main structural differences?
Determine the number of possible stereoisomers for ketooctose.
True or False. A ketohexose has 4 asymmetric carbons and 16 stereoisomers, while an aldohexose has 3 asymmetric carbons and 8 stereoisomers.
Identify the given monosaccharide as D or L.
Draw the Fischer projections of the following aldohexoses.
i. L-Mannose
ii. L-Galactose
Consider the following line-angle drawing of a molecule.
Convert this into a correct Fischer projection using appropriate bond rotations.
Galactose is the C4 epimer of glucose. Draw the structure of D-galactose in
a. Fischer projection.
b. Chair conformation of the β-pyranose anomer.
c. Haworth projection.