- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

Diazoxide is a medication prescribed to treat low blood pressure. The molecule below has a similar structure to diazoxide and can be synthesized as shown below. Suggest a mechanism of this reaction.
The following is a synthesis of an α-hydroxy acid from an amino acid. Provide the mechanism for this reaction, starting with the diazonium ion intermediate. (Note that the alcohol oxygen is the same in both the reactant and the product).
Amino acids are α-aminocarboxylic acid compounds. For the given amino acid, determine the carbonyl compound needed to synthesize it.
Show how the following conversions can be accomplished.
a. N-ethyl-1-phenylethanamine from acetophenone
b. 1-benzylpyrrolidine from benzaldehyde
c. N-ethylaniline from aniline
A common method to produce amines is the reductive amination of aldehydes and ketones. Show how the following synthesis can be accomplished using this technique.
- benzaldehyde → methylbenzylamine
Draw the structure of the major product expected from the exhaustive methylation and treatment with Ag2O followed by heating of pentan-2-amine.
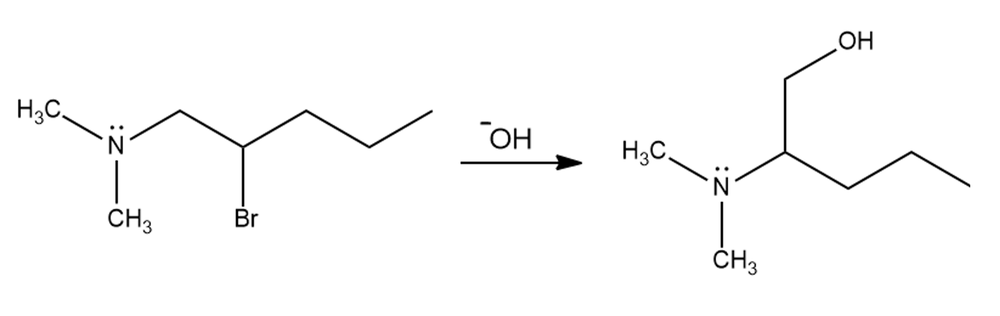
For the following reaction, explain why the carbon bonded to the bromo group in the reactant is not attached to the hydroxyl group in the product.
Give the expected major products when N,N-diethylcyclopentanamine is treated with hydrogen peroxide and heated.
Draw the expected products from the reaction of the following tertiary amines with H2O2 followed by heat.

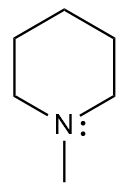
What is the compound produced for the reaction of the given tertiary amine with hydrogen peroxide and heat?