- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

Predict the product of the reaction given below.

Show how the below-given compound can be produced using an alkyl halide and a ketone.

When an α-haloketone reacts with the hydroxide ion, a carboxylic acid is formed. This is known as a Favorskii reaction. Propose a suitable mechanism for the Favorskii reaction below. Hint: The first step is deprotonation of the α-carbon that is not bonded to Br, the second step is forming a three-membered ring, and in the third step HO− acts as a nucleophile.

Draw the structures of the major products expected from the reaction given below.

Provide the complete equations of the following reactions showing the alkylated and acylated intermediates and the final products expected after their hydrolysis.
a. piperidine enamine of propan-2-one + allyl bromide
b. piperidine enamine of propan-2-one + acetyl chloride
Consider the following enamine alkylation reaction:
diethylamine enamine of cyclohexanone + iodoethane
Write the equation showing the expected product of the alkylation reaction and provide the final products expected after hydrolysis of the iminium salt.
Show how the following conversion can be achieved using any necessary reagents.

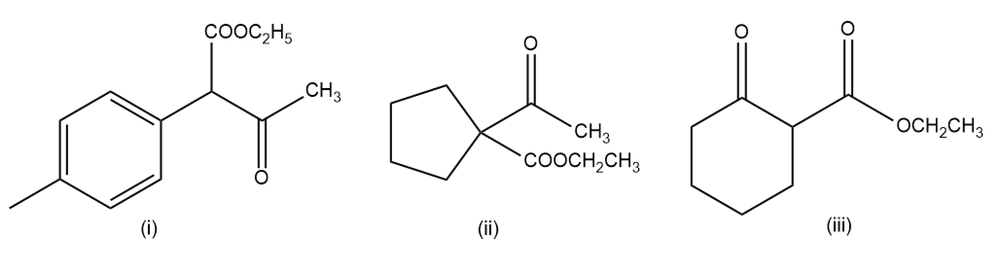
Show the ketones produced after the hydrolysis and decarboxylation of the following β-keto esters.
Predict the alkyl halide required for the acetoacetic ester synthesis of the given compound.
Create a synthesis for the molecule shown below starting from the given compound.

What carboxylic acid is formed in the following malonic ester synthesis?
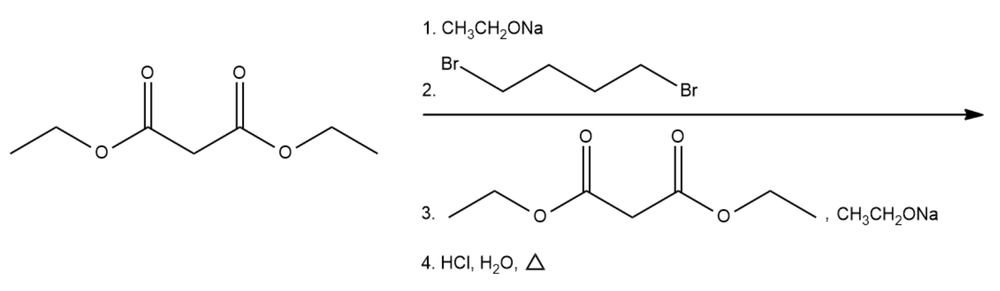
Provide suitable reactions to produce the following compounds using malonic ester synthesis.