- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

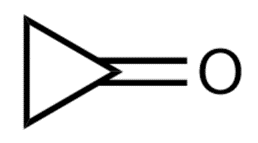
Which of the following para-substituted benzaldehydes is most likely to be found in the hydrate form in an aqueous solution?

Arrange the given compounds in increasing order of their equilibrium constant for hydration (lowest to highest).
Name the functional group present in the following compounds and show the products of their complete hydrolysis.
Show how the following acetals can be produced using suitable carbonyl compounds and alcohols.
Name the functional group present in the following compounds and show the products of their complete hydrolysis.
Draw the structure of the product formed when benzaldehyde reacts with ethylene glycol in an acidic medium.
It is possible to selectively hydrogenate alkenes with the presence of ketones in the same molecule. Supposing that was not the case, suggest a strategy to generate (i) from (ii) with the use of a protecting group.

Identify the starting materials (including a carbonyl compound) required to form the following structure:

Acetals are used as protecting groups for carbonyl compounds (aldehydes and ketones) and diols. A cyclic acetal is formed when a ketone or an aldehyde reacts with a diol. Determine the cyclic acetal produced when the compound below reacts with ethane-1,2-diol under acid catalysis.
Show what amines and carbonyl compounds that produce the imines listed below.
Draw the structures of the major products expected from the reactions given below.
Propose a mechanism to show how cyclopentanone reacts with piperidine in the presence of an acid.
Draw the carbonyl compound and the amine that would combine to produce the derivative below.