- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

Write the IUPAC name for the compound given below.
Name the following compound. Give both an IUPAC and a common name if possible.
- CH3CH=CHCHO
Provide the IUPAC name of the following compounds.

Name the following compound. Give both an IUPAC and a common name if possible.
Name the following compound. Give both an IUPAC and a common name if possible.
- CH3COCH2CH3
Rationalize why the carbonyl oxygen of the ester attacks the aluminum of DIBAL-H instead of the alkoxy oxygen in the following reaction.

Show how 1-bromopentane can be converted to hexanal. Provide any necessary reagents.
(i) Predict the main product obtained when the molecule below reacts with DIBAl-H.
(ii) Based on your answer, is there a need to convert the ester to another ester to accomplish what DIBAl-H is intended to do?

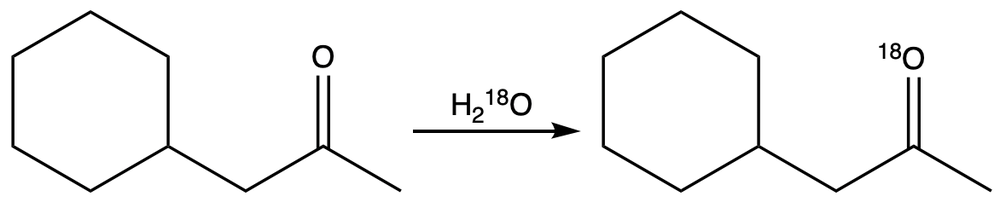
It is observed that when 18O-labeled water dissolves a ketone, the oxygen in it is incorporated into the ketone. Propose the plausible mechanism for the reaction.
Is the formation of a cyanohydrin possible by adding a ketone to a solution of KCN?
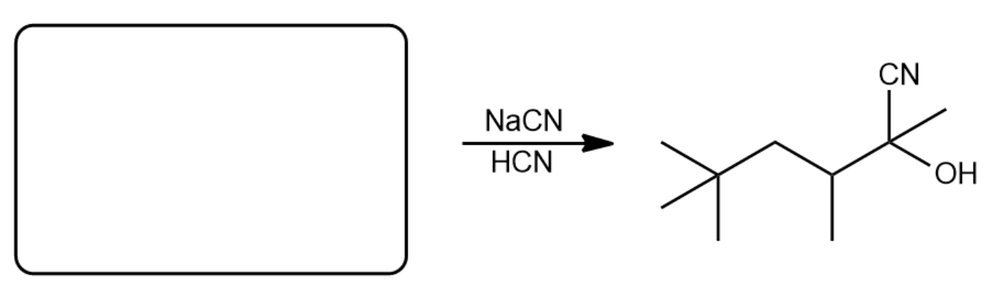
Determine the carbonyl compound to react with NaCN/HCN to yield the cyanohydrin below.
Identify the secondary alcohol produced when cyclopentyl formate reacts with an excess amount of Grignard reagent.
Draw the products obtained by the reaction between cyclopentanecarbaldehyde and the reagents shown below.
CH3MgBr, then H3O+
i) Draw a mechanism for each of the reactions. ii) Which of the two reactions would be considered more sustainable? Note: Assume that the starting organic molecules are equally green.