- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

In the Diels-Alder reaction shown below, a Lewis acid-Lewis base complex forms with the dienophile. Predict what will happen to the rate of the reaction and justify your answer.

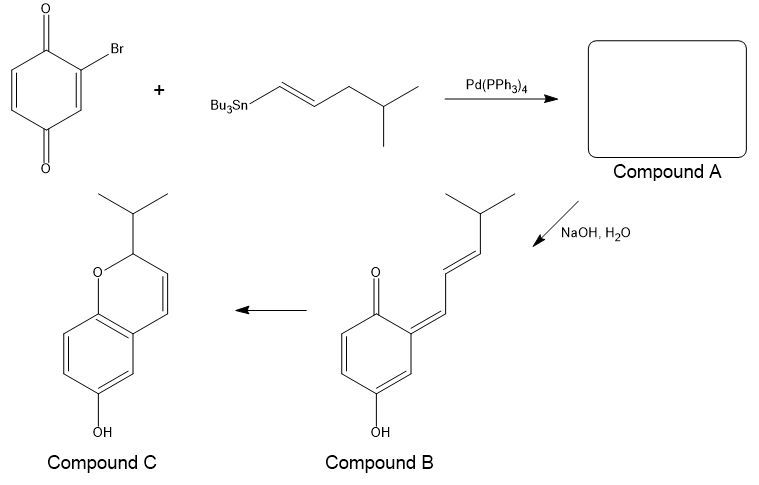
The first step of the following series of reactions is Stille coupling. In a basic solution, the product (A) of the first step tautomerizes to produce compound B, and compound B is immediately converted to compound C.
(a) Draw the structure of compound A.
(b) Propose a mechanism for the formation of compound B from compound A.
Provide a general principle that may be utilized in anticipating the main product of a Diels-Alder reaction involving an alkene that has a substituent that can withdraw electrons and a diene that has a substituent that, based on its placement, can donate electrons through resonance.
In the following Diels–Alder reactions, predict the final products. Include stereochemistry, if applicable.

Draw the structures of the major products in the given chemical reactions.

Provide the final product expected from the Diels–Alder reaction given below. Show stereochemistry if required.

Determine the dienes and dienophiles required to form each of the products given below.
Determine the dienes and dienophiles required to form each of the products given below.

Arrange the following compounds in decreasing order of λmax.

Identify the molecule that will most likely absorb light in the visible region.

Determine whether the following statement is true or false. If false, correct the statement.
A conjugated alkene with a symmetric HOMO has an odd number of double bonds.
Write the appropriate electronic configuration for the following compound.
octa-1,3,5,7-tetraene (ground state of pi electrons system)
Draw the molecular orbitals for cycloheptatrienyl ions and their energy diagram. Draw all bonding MOs and all the pairs of degenerate MOs. Label each orbital as bonding, nonbonding, or antibonding.
Give a two-step reaction for the conversion of the reactant to the product.