- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

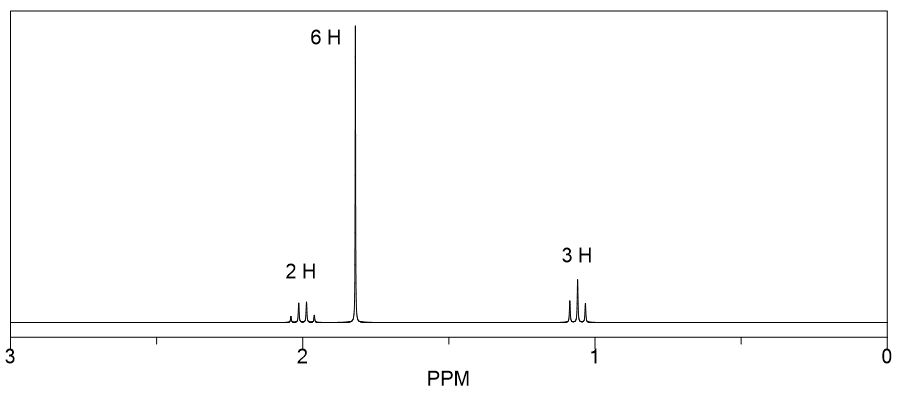
Which of the three isomers with the molecular formula C6H11Cl has the following 1H NMR spectrum?

Draw the DEPT sequence spectra expected for the molecule given below.


Draw the expected 13C NMR spectrum for the given molecule.

A student tried to synthesize some cyclohexyl chloride in the laboratory by reacting cyclohexanol with one equivalent of sodium chloride in a large excess of concentrated sulfuric acid. Unfortunately, the synthesis was not successful, and after the synthesis was completed, the student isolated a compound of formula C6H10. If the 13C-NMR spectrum of the compound is shown below:
(i) Give a plausible structure for the product.
(ii) Assign all peaks in the 13C-NMR spectrum of the product.
(iii) To obtain a higher yield of cyclohexyl chloride, suggest modifications in the reaction.
There are two possible McLafferty rearrangements that 2-methyloctan-4-one can undergo. What products are formed in each case?

Which compound produces the most intense peak at m/z = 71?
The mass spectra of 1-methoxybutane and 1-ethoxypropane are shown below. Identify which spectrum corresponds to each compound


Use the rule of 13 to provide the molecular formula of a compound that contains C, H, and two O with M+ = 118.
Determine the possible number/s of N in a neutral compound that contains C, H, and N with M+ = 84.
A high-resolution mass spectrometer can distinguish between C7H10N2 and C7H6O2, both having a molecular mass of 122 amu. True or false.
Apart from using the molecular mass, how else can you determine that the fragment peak at m/z 63 in the below-given mass spectrum for 1-bromo-2-chloroethane includes chlorine, not bromine?

What is the expected ratio of the M, M + 2, and M + 4 peaks for 1,2-dibromoethane?

Consider the following mass spectrum:

Identify which distinctive feature indicates the presence of sulfur, chlorine, bromine, iodine, or nitrogen. Try to determine the molecular formula of the compound.