- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

Determine the expected number of signals in the 1H NMR spectrum of the compound below.

How many different NMR signals will be observed in the NMR spectrum of each of the following compounds theoretically? Determine the approximate chemical shift for each proton and highlight any diastereotopic relationships.
(i) PhCHClCH2Cl
(ii) vinyl bromide
What are the expected chemical shifts (ppm) for each of the protons present in the structures given below?
Compound A, with a molecular formula C3H4, shows two singlets in its 1H NMR spectrum (with a ratio 3 : 1). Compound B is formed when compound A undergoes an acid-catalyzed hydration. Compound B also gives a positive iodoform test, and shows a singlet in its 1H NMR spectrum. Determine the structures A and B.
For the hydrogen indicated in red, sketch the expected 1H NMR signal showing its estimated chemical shift.

Fill out the values in the 1H NMR table given below for the indicated hydrogens in the following compound.


Provide the integration for the 1H NMR signals expected for the hydrogens at the indicated carbons in the given compound. (Hint: Observe if there is symmetry in the structure.)

Give the predicted number of signals and the relative ratio of signal integrations for the molecule depicted below.

Draw the expected signal for Ha in the below-given molecule with the help of given values.

A chemist observes a doublet with a coupling constant (J) value of 6.6 Hz and a chemical shift (δ) about 3.00 ppm in the NMR spectrum of a compound measured using a 60.0-MHz spectrometer.
(i) If the spectrum is taken using a 300-MHz spectrometer, what would the chemical shift (δ) be?
(ii) What would the splitting value J be if the spectrum is taken using a 300-MHz spectrometer?
(iii) How many Hz from the TMS peak is this signal in the 60.0-MHz spectrum? In the 300-MHz spectrum?
For the following molecule, illustrate the splitting diagram for proton (Hb), given that the Jbc = 12 and Jba = 4.

For the given compound, draw an estimated 1H NMR spectrum, and show the multiplicity, integration, and chemical shift of each signal. Mark the signals according to the sets of equivalent hydrogens.
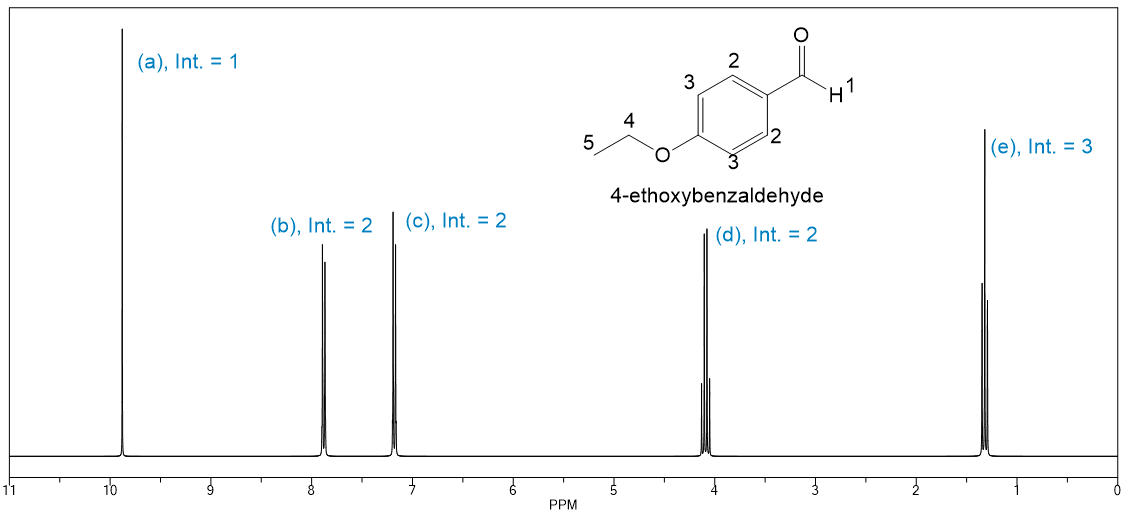
Match the peaks in the 1H NMR spectrum of the given molecule with their corresponding hydrogens.