15. Analytical Techniques:IR, NMR, Mass Spect - Part 2 of 4
15. Analytical Techniques:IR, NMR, Mass Spect / 1H NMR:Q-Test / Problem 6
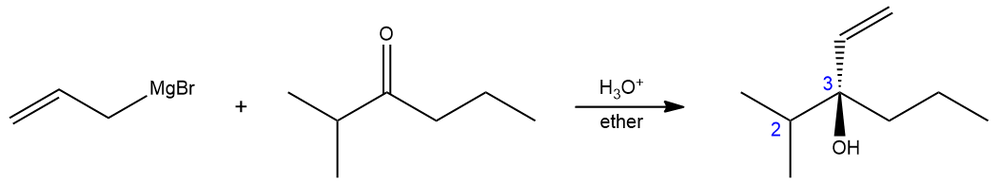
In an organic chemistry lab experiment, 2-methyl-3-hexanone is reacted with allyl magnesium bromide yielding a tertiary alcohol. The 1H NMR of 2-methyl-3-hexanone shows that the two methyl groups are equivalent indicated by a one doublet peak. On the other hand, the product (a racemic mixture), gives two different 3H doublets.
(i) Along the C2–C3 axis, show a Newman projection of the product.
(ii) Why do the methyl groups of the product show different signals? What is the term used to describe such groups?

Learn this concept