- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

Determine the magnetic field strength necessary when a 750 MHz instrument for 1H NMR is used. (γ for 1H = 2.675 × 108 T-1s-1)
How much do two signals differ in a 500 MHz spectrometer, if in a 400 MHz spectrometer they differ by 3.5 ppm?
Determine if each of the following statements is true or false:
i. The coupling constant in hertz (Hz) is directly proportional to the operating frequency.
ii. Compared to the operating frequency in IR and UV-vis spectroscopy, the operating frequency in NMR spectroscopy is much higher.
Write down how many signals are there and indicate their multiplicity in the 1H NMR spectra of the given molecule.
(CH3)3CCH2CH2CH2CH2CH2CH2C(CH3)3
The following figure shows that proton H a will appear as a doublet in the 1H NMR spectrum based on the ratio of possible spin arrangements of Ha and Hb. How would Hb appear in the spectrum?

Determine the splitting patterns in the 1H NMR spectra for the molecule given below:

Which of the two bonds, C—H or C—N, will vibrate at a higher wavenumber?
Given that the force constant is identical for C–C, C–F, C–O, and C–Br bonds, arrange the stretching frequency of the bonds from lowest to highest.
Identify which of the following bonds would show IR-active vibrations and which would not.
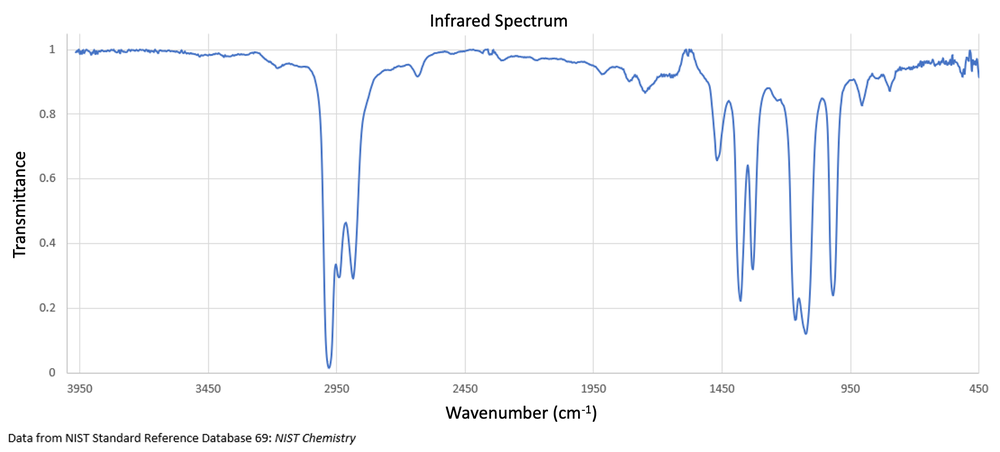
Determine the functional group present in a compound that has a chemical formula of C6H14O and an IR spectrum displayed below.
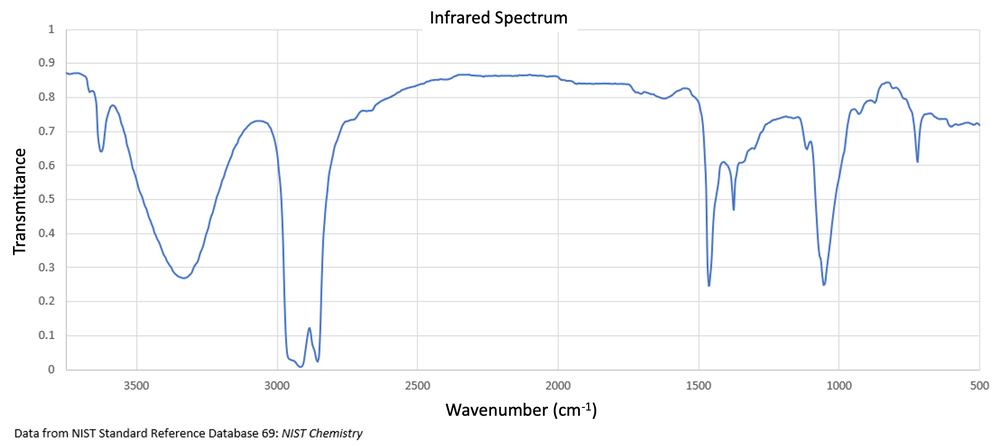
Decanal is reduced to 1-decanol by using sodium borohydride. How does the IR spectrum shown below confirm the formation of the alcohol?
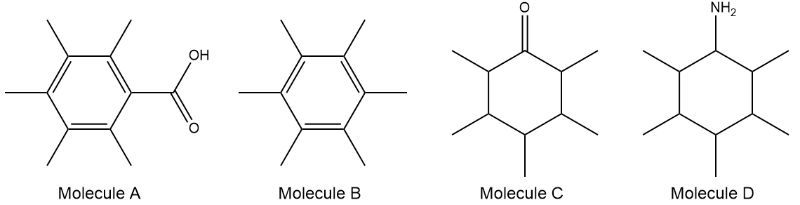
Match the IR spectrum to the correct molecule.

Predict the important peaks present in the IR spectrum of the molecule shown below.