- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

Provide two different pathways to carry out the following reaction.

Determine the alkene molecule needed to be reacted with a peroxyacid to produce the following epoxide.
(i) cyclopentene oxide
(ii) 2-isopropyloxirane
Show how tetramethylethylene oxide will react when treated with each of the following reagents.

(a) NaOCH2CH3 (sodium ethoxide)
(b) NaNH2 (sodium amide)
(c) NaSPh (sodium thiophenoxide)
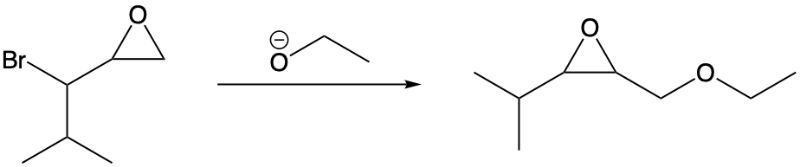
What is the reaction mechanism for the following reaction?
Propose a mechanism for the following acid-catalyzed double cyclization. Hint: The mechanism looks like the cyclization of squalene oxide to lanosterol.

Predict the major products (including stereochemistry if applicable) formed from the following reactions.
(i) (2S,3R)-2,3-diethyl-2-methyloxirane + CH3CH2O–/CH3CH2OH
(ii) (2S,3R)-2,3-diethyl-2-methyloxirane + H+/CH3CH2OH
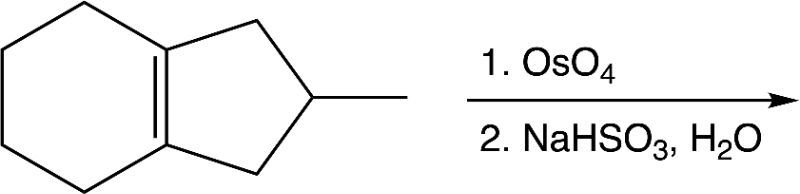
Determine the product(s) for the given reaction below.

Indicate the stereochemistry, and draw both enantiomers if the product is a racemic mixture.
What are the products of the reaction? Draw both enantiomers if the product is a racemic mixture.
Show the products that will form when the following alkene reacts as follows:

Predict the products when the given compounds are treated with ozone and then with dimethyl sulfide.
When an unknown compound was allowed to react with ozone, followed by dimethyl sulfide and a small amount of water, it produced acetic acid, 3-methylbutan-2-one, and 3-oxopenatanoic acid. Determine the structure of the unknown compound. Which information can not be determined with this reaction?
Draw the products formed when the compounds below react with 1) O3 -78°C, 2) (CH3)2S.

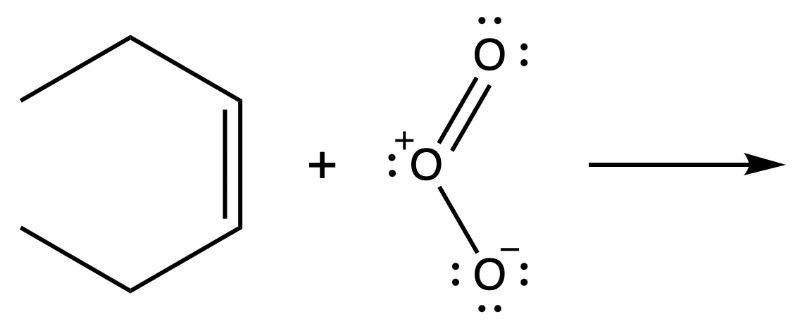
For the reaction shown, draw the mechanism.