- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

Propose a synthesis for the compound shown below using 1-methylcyclopent-1-ene as the starting molecule.

Show how to accomplish the synthetic conversion shown below.
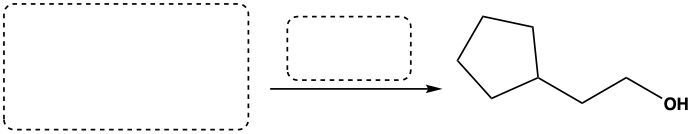
Determine the starting materials that are needed to synthesize the following alcohol.
Identify the products of the hydroboration–oxidation reaction of the following compounds.
(i) cyclopentene
(ii) 1-isopropylcyclopent-1-ene
Label each chiral center as R or S.
For each of the following reactions, determine the major products. Include stereochemistry in your answer.

Draw the product when partial hydrogenation (H2, Pd) is performed on the molecule arachidonic acid.

Determine the products that will form in the following reaction conditions.

What are the products formed in the halogenation reaction given below? Indicate the stereochemistry of the products and any racemic mixtures.

Propose a mechanism and predict the major products of the following reactions. Include stereochemistry in your answers where appropriate.
(i) (E)-pent-2-ene + Br2 in CCl4
(ii) (Z)-pent-2-ene + Br2 in CCl4
Predict the products formed in the following reactions:

Illustrate the product(s) formed when the given compound undergoes a reaction with Br2 and EtOH.

Provide an arrow-pushing mechanism for the given reaction that explains how the halohydrin is formed in a regioselective and stereospecific manner.

Identify the major product formed in the reaction given below.