- Download the worksheet to save time writing
- Start solving the practice problems
- If you're stuck, watch the video solutions
- See your summary to get more insights

For the molecular formulas given below, give the number of elements of unsaturation. Select the appropriate structure as an example for each.
(i) C6H7NO2
(ii) C7H5BrIN

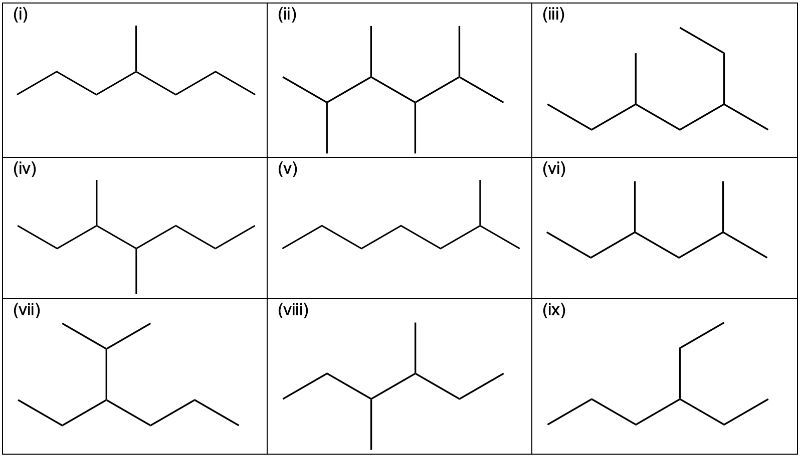
Which of the following are constitutional isomers of an alkane with a molecular formula of C8H18?
Draw the structural isomers with the molecular formula C4H8 that do not show cis/trans isomerism and have a carbon-carbon double bond.
Show all 4 acyclic isomers with the formula C3H5Cl. (Note: If applicable, include stereoisomers.)
Determine all the pushable electron pairs and the regions where they can be pushed in the structure below. Also, provide one valid resonance structure.

Draw all the resonance contributors of the given species and predict their relative stabilities. Also, draw its resonance hybrid.

Provide the resonance forms of the radical shown below.

Determine the appropriate hybridization of all indicated atoms in the following organic compounds.

Determine the hybridization of the carbon atoms.

What are the hybridizations and molecular geometries around the highlighted atoms?

Predict the molecular geometry and the Lewis structure of HCO 2–.
Keeping the resonance in mind, suggest the hybridization and geometry of the central atoms (only carbon and nitrogen) in the given species.

Select all sets that correctly describe the Lewis structure, the contribution of bond dipole moments and non-bonding pairs of electrons to the molecular dipole moment, and an estimation of the magnitude of the dipole moment for compounds CH2=NCH3, CH3OH and CCl4.
(1) Small molecular dipole moment. (2) Small molecular dipole moment.


(3) Large molecular dipole moment. (4) Small molecular dipole moment.


(5) Zero molecular dipole moment.