Here are the essential concepts you must grasp in order to answer the question correctly.
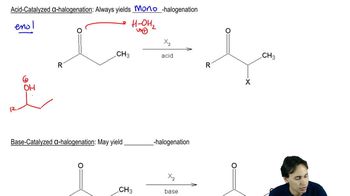
Acid-Catalyzed Halogenation
Acid-catalyzed halogenation involves the addition of halogens to carbonyl compounds in the presence of an acid catalyst. This reaction typically leads to the formation of α-halo ketones, which can undergo further transformations. The acid activates the carbonyl, making it more electrophilic and facilitating the nucleophilic attack by the halogen.
Recommended video:
α,β-Unsaturated Ketones
α,β-Unsaturated ketones are compounds that contain a carbon-carbon double bond between the α and β carbons relative to the carbonyl group. These structures are important intermediates in organic synthesis, particularly in Michael reactions, where they serve as Michael acceptors. Their reactivity is influenced by the conjugation of the double bond with the carbonyl, enhancing electrophilicity.
Recommended video:
Michael Reaction
The Michael reaction is a nucleophilic addition reaction where a nucleophile adds to an α,β-unsaturated carbonyl compound. This reaction is significant in forming carbon-carbon bonds and is widely used in synthetic organic chemistry. The nucleophile typically attacks the β-carbon, leading to the formation of a new carbon-carbon bond and resulting in a more complex molecule.
Recommended video: