Here are the essential concepts you must grasp in order to answer the question correctly.
Cyclohexane Chair Conformation
The cyclohexane chair conformation is a three-dimensional arrangement of cyclohexane that minimizes steric strain and torsional strain. In this conformation, the carbon atoms are arranged in a staggered manner, allowing for more stable interactions between hydrogen atoms. The chair form can flip, interchanging axial and equatorial positions of substituents, which is crucial for understanding the stability of different isomers.
Recommended video:
What is a chair conformation?
Axial and Equatorial Bonds
In the chair conformation of cyclohexane, substituents can occupy two types of positions: axial and equatorial. Axial bonds are oriented perpendicular to the plane of the ring, while equatorial bonds extend outward from the ring's plane. The positioning of substituents affects steric interactions and overall stability, with equatorial positions generally being more favorable for larger groups due to reduced steric hindrance.
Recommended video:
Chair Flip Mechanism
The chair flip mechanism refers to the process by which cyclohexane interconverts between two chair conformations. During this flip, all axial bonds become equatorial and vice versa, allowing for the redistribution of substituents. This concept is essential for understanding the dynamic nature of cyclohexane and how substituent positions can influence the molecule's reactivity and stability.
Recommended video:
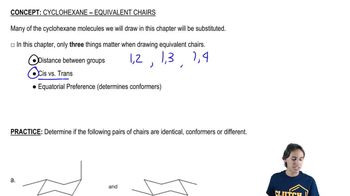
The 3 important factors when drawing chairs
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution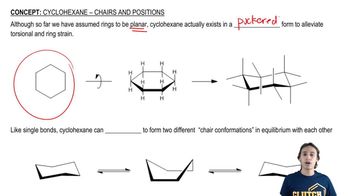


 1:18m
1:18m