Here are the essential concepts you must grasp in order to answer the question correctly.
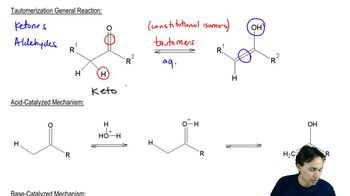
Enol Formation
Enol formation involves the conversion of a carbonyl compound, such as a ketone or aldehyde, into its corresponding enol form, which features a double bond between carbon and an alcohol group. This process is crucial in organic chemistry as enols can participate in various reactions, including tautomerization and electrophilic addition.
Recommended video:
Acid-Catalyzed Mechanism
In acid-catalyzed mechanisms, the presence of an acid facilitates the reaction by protonating the carbonyl oxygen, increasing the electrophilicity of the carbonyl carbon. This step is essential for the formation of the enol, as it allows for the subsequent rearrangement and deprotonation to yield the enol form.
Recommended video:
Acid-catalyzed hydration mechanism
Tautomerization
Tautomerization is a chemical equilibrium between two isomers, typically involving the migration of a hydrogen atom and a shift of a double bond. In the case of phenylacetone, the keto form can convert to the enol form through tautomerization, which is a key concept in understanding the stability and reactivity of enols in various chemical contexts.
Recommended video:
Tautomerization Mechanisms
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 1:51m
1:51m