Here are the essential concepts you must grasp in order to answer the question correctly.
Epimers
Epimers are a specific type of diastereomer that differ in configuration at only one stereogenic center. In carbohydrates, this means that two sugars can have the same molecular formula and differ only at one carbon atom's stereochemistry. Understanding epimers is crucial for distinguishing between similar sugars, such as D-allose and D-talose, which are epimers of glucose and D-galactose, respectively.
Recommended video:
Identifying Types of Stereoisomers
Fischer Projections
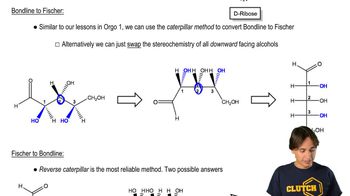
Fischer projections are a two-dimensional representation of three-dimensional organic molecules, particularly useful for depicting the stereochemistry of sugars. In these projections, vertical lines represent bonds that go back into the plane, while horizontal lines represent bonds that come out of the plane. Mastery of Fischer projections is essential for accurately drawing and interpreting the structures of carbohydrates like D-allose and D-talose.
Recommended video:
Monosaccharides - Drawing Fischer Projections
D and L Configuration
The D and L configuration refers to the orientation of the hydroxyl group on the chiral carbon farthest from the carbonyl group in a sugar molecule. In D-sugars, this hydroxyl group is on the right in Fischer projections, while in L-sugars, it is on the left. Recognizing the D and L configurations is vital for correctly identifying and drawing the structures of sugars such as D-allose and D-talose.
Recommended video:
Representations of L-Configuration
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: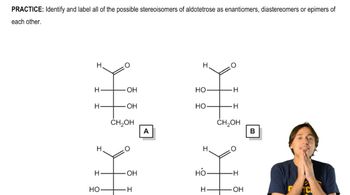


 2:57m
2:57m