Here are the essential concepts you must grasp in order to answer the question correctly.
Constitutional Isomers
Constitutional isomers are compounds that have the same molecular formula but differ in the connectivity of their atoms. This means that the arrangement of atoms in the molecule varies, leading to different structural formulas. Understanding constitutional isomers is crucial for determining the various forms a compound can take, which is essential for the question regarding C4H10O.
Recommended video:
What is a constitutional isomer?
Functional Groups
Functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. In the case of C4H10O, the presence of an alcohol (-OH) or ether (R-O-R') functional group can lead to different isomeric forms. Recognizing these functional groups helps in identifying the types of isomers that can be formed.
Recommended video:
Identifying Functional Groups
Structural Representation
Structural representation refers to the way in which the arrangement of atoms in a molecule is depicted, often through structural formulas or skeletal structures. This representation is vital for visualizing and distinguishing between different constitutional isomers. For C4H10O, drawing out the various structures will aid in identifying all possible isomers and understanding their unique properties.
Recommended video:
Representations of L-Configuration
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution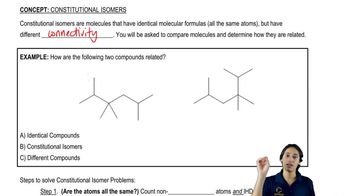



 1:10m
1:10m