Here are the essential concepts you must grasp in order to answer the question correctly.
Hydrogen Bonding
Hydrogen bonding is a strong type of dipole-dipole interaction that occurs when a hydrogen atom covalently bonded to a highly electronegative atom, like oxygen, interacts with another electronegative atom. In 1,4-dihydroxybenzene, the hydroxyl groups are positioned para to each other, allowing for effective hydrogen bonding between molecules, which increases the melting point due to the additional energy required to break these interactions.
Recommended video:
The definition of hydrogenation.
Molecular Structure and Symmetry
The molecular structure and symmetry of a compound significantly influence its physical properties, including melting point. 1,4-dihydroxybenzene has a symmetrical structure that allows for optimal packing in the solid state, leading to stronger intermolecular forces. In contrast, 1,2-dihydroxybenzene has a less symmetrical arrangement, resulting in weaker interactions and a lower melting point.
Recommended video:
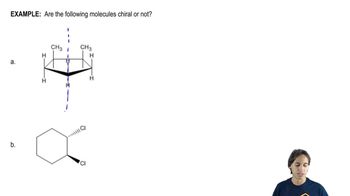
Determining Chirality with Plane of Symmetry
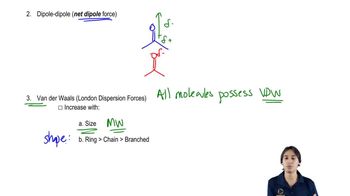
Intermolecular Forces
Intermolecular forces are the forces of attraction between molecules, which play a crucial role in determining the physical properties of substances. In the case of 1,4-dihydroxybenzene, the presence of strong hydrogen bonds and effective packing leads to higher intermolecular forces compared to 1,2-dihydroxybenzene, which has weaker van der Waals forces. This difference in intermolecular forces is a key reason for the disparity in melting points.
Recommended video:
How Van der Waals forces work.