Here are the essential concepts you must grasp in order to answer the question correctly.
Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR spectroscopy is a powerful analytical technique used to determine the structure of organic compounds. It relies on the magnetic properties of certain nuclei, primarily hydrogen (1H) and carbon (13C), to provide information about the environment of these atoms in a molecule. The resulting spectra reveal chemical shifts and splitting patterns that help identify the number of neighboring hydrogens and their arrangement.
Recommended video:
Spin-Spin Coupling
Spin-spin coupling, or J-coupling, occurs when non-equivalent neighboring hydrogen atoms influence each other's magnetic environments, leading to splitting of NMR signals. The number of peaks in a signal is determined by the n+1 rule, where n is the number of neighboring hydrogens. This concept is crucial for predicting the splitting patterns observed in NMR spectra, allowing chemists to deduce the connectivity of atoms in a molecule.
Recommended video:
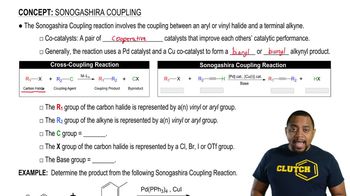
Sonogashira Coupling Reaction
Chemical Environment
The chemical environment refers to the specific surroundings of an atom within a molecule, which affects its electronic and magnetic properties. Factors such as electronegativity of nearby atoms, hybridization, and steric effects can alter the chemical shift and splitting patterns observed in NMR. Understanding the chemical environment is essential for accurately predicting how hydrogens will split in the spectrum, as it directly influences the observed NMR signals.
Recommended video:
Chemical Reactions of Phosphate Anhydrides Concept 1
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 12:21m
12:21m