Here are the essential concepts you must grasp in order to answer the question correctly.
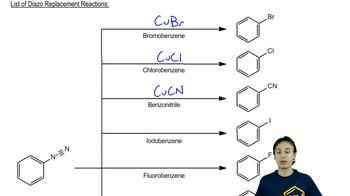
Diazotization
Diazotization is a chemical reaction that involves converting an amine group (-NH2) into a diazonium salt (-N2+). This is typically achieved by treating the amine with sodium nitrite (NaNO2) in the presence of a strong acid like hydrochloric acid (HCl). The resulting diazonium salt is a key intermediate in various organic reactions, allowing for further transformations such as substitution reactions.
Recommended video:
Nucleophilic Aromatic Substitution
Nucleophilic aromatic substitution (NAS) is a reaction where a nucleophile replaces a leaving group on an aromatic ring. In this context, the diazonium salt generated from the amine can undergo substitution with a nucleophile, such as bromide ions (Br-) from CuBr. This reaction is facilitated by the electron-withdrawing nitro group, which stabilizes the negative charge developed during the reaction.
Recommended video:
Nucleophiles and Electrophiles can react in Substitution Reactions.
Reactivity of Diazonium Salts
Diazonium salts are highly reactive intermediates in organic chemistry, particularly in electrophilic aromatic substitution reactions. They can easily undergo substitution with various nucleophiles, including halides, phenols, and other aromatic compounds. The stability of the diazonium salt and the nature of the substituents on the aromatic ring significantly influence the reaction pathway and the products formed.
Recommended video: