Here are the essential concepts you must grasp in order to answer the question correctly.
Types of Chemical Bonds
Chemical bonds can be classified into sigma (σ) and pi (π) bonds. Sigma bonds are formed by the head-on overlap of atomic orbitals, allowing for free rotation around the bond axis. In contrast, pi bonds result from the side-to-side overlap of p orbitals and restrict rotation. Understanding these bond types is crucial for analyzing reactions, as they dictate the stability and reactivity of molecules.
Recommended video:
Chemical Reactions of Phosphate Anhydrides Concept 1
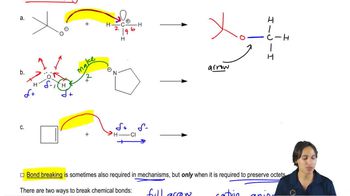
Bond Breaking and Formation
In a chemical reaction, bonds in the reactants must break to allow for the formation of new bonds in the products. The process of bond breaking requires energy input, while bond formation releases energy. Identifying which bonds are broken and formed helps in understanding the reaction mechanism and energy changes involved, which are essential for predicting reaction outcomes.
Recommended video:
Identifying Bond Breaking
Reaction Mechanisms
A reaction mechanism describes the step-by-step sequence of elementary reactions by which overall chemical change occurs. It provides insight into how bonds are broken and formed during the reaction. Understanding the mechanism is vital for predicting the products and understanding the kinetics and thermodynamics of the reaction, which are key for effective analysis in organic chemistry.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 7:16m
7:16m