Here are the essential concepts you must grasp in order to answer the question correctly.
Toxicity of Organohalides
Methyl iodide and methyl bromide are both organohalides, but their toxicity can differ due to the nature of the halogen atom. Iodine is generally more electronegative than bromine, leading to stronger interactions with biological systems, which can result in higher toxicity. Additionally, the molecular structure and reactivity of methyl iodide may allow it to disrupt biological processes more effectively than methyl bromide.
Recommended video:
How to recognize alcohols, amines and ethers.
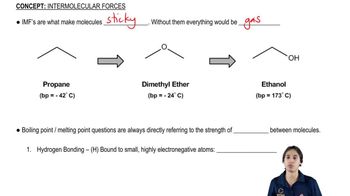
Volatility and Boiling Points
The boiling point of a compound is a key factor in its volatility, which influences how easily it can evaporate and enter the atmosphere. Methyl bromide has a lower boiling point (4 °C) compared to methyl iodide (43 °C), making it more volatile and likely to escape into the atmosphere during application. This higher volatility contributes to methyl bromide's greater potential to reach the stratosphere and affect the ozone layer.
Recommended video:
How IMFs are related to melting and boiling points.
Atmospheric Lifetime and Degradation
The atmospheric lifetime of a compound refers to the duration it remains in the atmosphere before being broken down by chemical reactions. Methyl bromide has a longer atmospheric lifetime than methyl iodide, which means it can persist longer and diffuse into the stratosphere. Methyl iodide, being less stable and more reactive, is likely to degrade more quickly in the lower atmosphere, reducing its chances of reaching the stratosphere.
Recommended video:
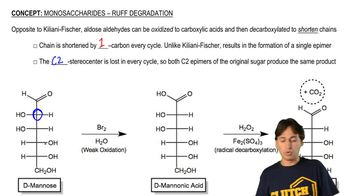
Monosaccharides - Ruff Degradation
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution

 0:58m
0:58m