Here are the essential concepts you must grasp in order to answer the question correctly.
Oxidation Reactions
Oxidation reactions involve the loss of electrons or an increase in oxidation state by a molecule. In organic chemistry, this often pertains to the addition of oxygen or the removal of hydrogen. In the context of sugars like D-mannose and D-galactose, oxidation can lead to the formation of carboxylic acids or other functional groups, significantly altering the properties and reactivity of the original compounds.
Recommended video:
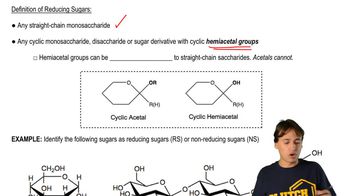
Structure of Sugars
D-mannose and D-galactose are both monosaccharides, specifically aldoses, which means they contain an aldehyde group. Understanding their structural formulas, including the arrangement of hydroxyl (-OH) groups and the anomeric carbon, is crucial for predicting the products of their oxidation. The stereochemistry of these sugars influences how they react with oxidizing agents like nitric acid.
Recommended video:
Nitric Acid as an Oxidizing Agent
Nitric acid (HNO3) is a strong oxidizing agent commonly used in organic chemistry to oxidize alcohols and aldehydes to carboxylic acids. When applied to sugars, it can oxidize the aldehyde group and potentially hydroxyl groups, leading to the formation of various oxidized products. The specific conditions of the reaction, such as concentration and temperature, can affect the extent and nature of the oxidation.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 3:10m
3:10m