Here are the essential concepts you must grasp in order to answer the question correctly.
D-Aldotetroses
D-Aldotetroses are a class of carbohydrates that contain four carbon atoms and an aldehyde functional group. They can exist in different stereoisomeric forms due to the presence of chiral centers. Understanding the structure and stereochemistry of these sugars is crucial for predicting their reactivity and the nature of the products formed during chemical reactions, such as oxidation.
Recommended video:
Monosaccharides - D and L Isomerism
Optical Activity
Optical activity refers to the ability of a compound to rotate the plane of polarized light, which is a characteristic of chiral molecules. When a molecule has one or more chiral centers, it can exist in two enantiomeric forms that are non-superimposable mirror images of each other. The formation of optically active aldaric acids from aldotetroses upon oxidation indicates that the resulting products retain chirality, which is essential for determining their optical properties.
Recommended video:
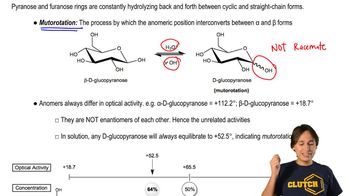
Mutorotation and Optical Activity
Oxidation with HNO3
Oxidation with nitric acid (HNO3) is a common reaction used to convert aldehydes and alcohols into carboxylic acids. In the case of aldotetroses, this reaction leads to the formation of aldaric acids, which are dicarboxylic acids derived from the oxidation of both the aldehyde and the primary alcohol functional groups. The specific aldotetroses that yield optically active aldaric acids upon oxidation will depend on their stereochemistry and the presence of chiral centers in the original sugar.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution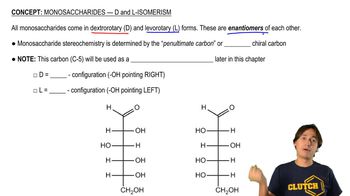


 3:10m
3:10m