Here are the essential concepts you must grasp in order to answer the question correctly.
Fischer Projection
The Fischer projection is a two-dimensional representation of a molecule, particularly useful for depicting the stereochemistry of carbohydrates. In this format, the vertical lines represent bonds that are oriented away from the viewer, while horizontal lines indicate bonds that are coming towards the viewer. This method allows for easy identification of the configuration of chiral centers in sugars, which is essential for converting to cyclic forms.
Recommended video:
Monosaccharides - Drawing Fischer Projections
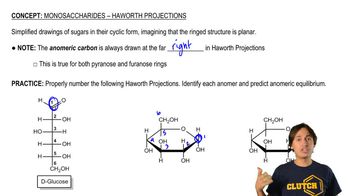
Haworth Projection
The Haworth projection is a way to represent the cyclic form of carbohydrates, showing the ring structure and the orientation of substituents. It provides a more realistic depiction of the three-dimensional shape of the molecule compared to Fischer projections. In this format, the ring is typically drawn as a planar structure, with substituents positioned above or below the plane, reflecting their actual spatial arrangement in solution.
Recommended video:
Monosaccharides - Haworth Projections
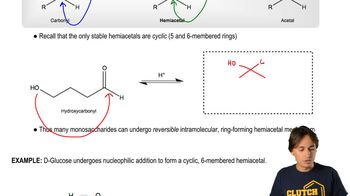
Cyclic Structure of Carbohydrates
Carbohydrates, such as D-mannose, can exist in both linear and cyclic forms. The cyclic structure arises from the reaction between a carbonyl group and a hydroxyl group within the same molecule, leading to the formation of a hemiacetal. This process is crucial for understanding the stability and reactivity of sugars, as the cyclic form is often more prevalent in biological systems and influences the molecule's properties and interactions.
Recommended video:
Monosaccharides - Forming Cyclic Hemiacetals
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution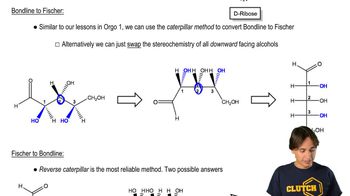

 4:03m
4:03m