Here are the essential concepts you must grasp in order to answer the question correctly.
Isomerism
Isomerism refers to the phenomenon where two or more compounds have the same molecular formula but different structural arrangements or spatial orientations. This can lead to variations in physical and chemical properties. Understanding isomerism is crucial for identifying and categorizing compounds, especially in organic chemistry, where the arrangement of atoms significantly influences reactivity and function.
Recommended video:
Monosaccharides - D and L Isomerism
Common Names vs. IUPAC Names
Common names are informal names used to identify chemical compounds, often based on historical or traditional usage, while IUPAC names are systematic names derived from established nomenclature rules set by the International Union of Pure and Applied Chemistry. Common names can sometimes be more recognizable but may not convey structural information as clearly as IUPAC names, making it important to understand both for effective communication in chemistry.
Recommended video:
The different parts of an IUPAC name
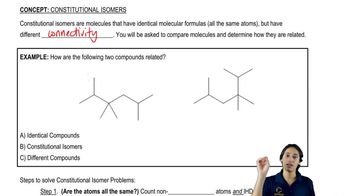
Types of Isomers
There are two main types of isomers: structural isomers, which differ in the connectivity of their atoms, and stereoisomers, which have the same connectivity but differ in the spatial arrangement of atoms. Within these categories, there are further subdivisions, such as geometric and optical isomers. Recognizing these types is essential for determining the number of isomers that can exist for a given compound, particularly when considering their common names.
Recommended video:
What is a constitutional isomer?
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution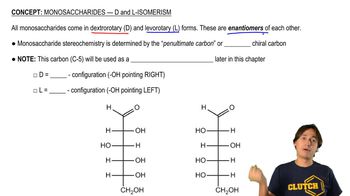


 6:42m
6:42m