Here are the essential concepts you must grasp in order to answer the question correctly.
Nitrile Structure and Reactivity
Nitriles are organic compounds containing a cyano group (-C≡N), which is a carbon triple-bonded to a nitrogen atom. They are polar and can act as electrophiles in reactions. When treated with Grignard reagents, nitriles undergo nucleophilic addition, where the nucleophile (from the Grignard reagent) attacks the electrophilic carbon of the nitrile, leading to the formation of an intermediate.
Recommended video:
Grignard Reagents
Grignard reagents are organomagnesium compounds represented as R-MgX, where R is an organic group and X is a halogen. They are highly reactive nucleophiles that can add to electrophilic centers, such as carbonyls and nitriles. In the reaction with nitriles, the Grignard reagent adds to the carbon of the cyano group, forming a tetrahedral intermediate that can further react to yield a ketone.
Recommended video:
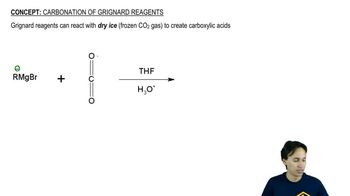
Carbonation of Grignard Reagents
Conversion to Ketone
The intermediate formed from the reaction of a nitrile with a Grignard reagent is a ketimine, which can be hydrolyzed to yield a ketone. This hydrolysis involves the addition of water, leading to the cleavage of the C-N bond and the formation of a carbonyl group (C=O). The overall transformation illustrates the utility of Grignard reagents in synthesizing ketones from nitriles.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 1:38m
1:38m