Here are the essential concepts you must grasp in order to answer the question correctly.
Acidity in Organic Compounds
Acidity in organic compounds refers to the ability of a molecule to donate a proton (H+) to a base. The strength of an acid is often determined by the stability of the resulting conjugate base; the more stable the conjugate base, the stronger the acid. Factors influencing acidity include electronegativity, resonance, and hybridization of the atom bearing the acidic proton.
Recommended video:
Resonance Stabilization
Resonance stabilization occurs when a molecule can be represented by two or more valid Lewis structures, allowing the electron density to be delocalized. This delocalization can stabilize the conjugate base formed after deprotonation, making the original compound more acidic. Aromatic compounds often exhibit significant resonance, which can enhance their acidity compared to aliphatic compounds.
Recommended video:
The radical stability trend.
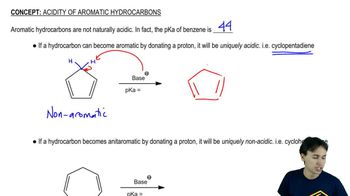
Aromaticity and Its Effects on Acidity
Aromaticity is a property of cyclic compounds that have a planar structure, complete delocalization of π electrons, and follow Huckel's rule (4n + 2 π electrons). Aromatic compounds tend to be more stable due to this delocalization, which can also influence their acidity. The presence of electron-withdrawing groups in an aromatic system can further enhance acidity by stabilizing the negative charge on the conjugate base.
Recommended video:
Aromatic hydrocarbon acidity

 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 5:39m
5:39m