Here are the essential concepts you must grasp in order to answer the question correctly.
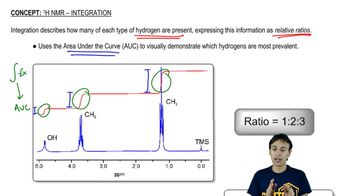
1H NMR Spectroscopy
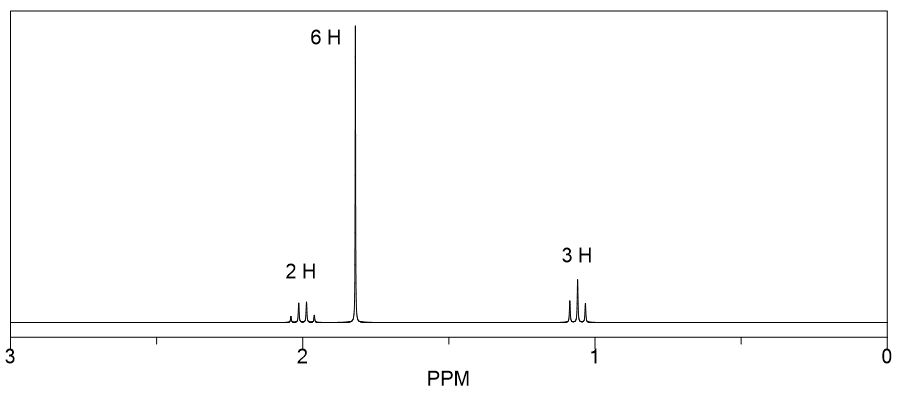
Proton Nuclear Magnetic Resonance (1H NMR) spectroscopy is a powerful analytical technique used to determine the structure of organic compounds. It provides information about the number of hydrogen atoms in different environments within a molecule, indicated by peaks in the spectrum. The chemical shift (measured in ppm) reveals the electronic environment of the protons, while the integration of peaks indicates the relative number of protons contributing to each signal.
Recommended video:
Isomerism
Isomerism refers to the phenomenon where compounds have the same molecular formula but different structural or spatial arrangements. In the case of C4H9Br, there can be several isomers, including structural isomers and stereoisomers. Understanding the different types of isomers is crucial for interpreting NMR spectra, as each isomer will produce a unique pattern of peaks based on its specific hydrogen environments.
Recommended video:
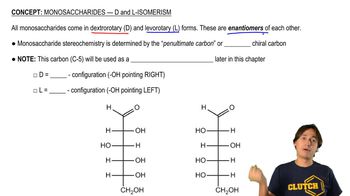
Monosaccharides - D and L Isomerism
Peak Integration and Splitting Patterns
In 1H NMR, peak integration provides the ratio of hydrogen atoms contributing to each signal, while splitting patterns (multiplicity) indicate the number of neighboring hydrogen atoms. For example, a singlet indicates no neighboring protons, while a doublet suggests one neighboring proton. Analyzing these patterns helps in deducing the connectivity and arrangement of atoms in the molecule, which is essential for identifying the correct isomer corresponding to the given spectrum.
Recommended video:
Common Splitting Patterns
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution