Here are the essential concepts you must grasp in order to answer the question correctly.
Chemical Shift in NMR Spectroscopy
Chemical shift in NMR spectroscopy refers to the resonance frequency of a nucleus relative to a standard in a magnetic field. It is influenced by the electronic environment surrounding the nucleus, which can be affected by electronegative atoms or functional groups. In organic compounds, shifts can indicate the presence of specific substituents, allowing for the identification of different molecules based on their unique chemical environments.
Recommended video:
pKa and Acidity
pKa is a measure of the acidity of a compound, representing the negative logarithm of the acid dissociation constant (Ka). A lower pKa value indicates a stronger acid, meaning it dissociates more readily in solution. The relationship between pKa and chemical shift arises because more acidic protons are often deshielded by electronegative groups, leading to downfield shifts in NMR spectra, which can help predict the acidity of different compounds.
Recommended video:
Electronegativity and Shielding Effects
Electronegativity refers to the tendency of an atom to attract electrons towards itself. In NMR, electronegative atoms can withdraw electron density from nearby protons, causing them to experience a stronger magnetic field and resulting in a downfield shift (higher ppm value). This concept is crucial for understanding how substituents on a molecule can influence both the chemical shift observed in NMR and the compound's acidity, as seen in the comparison of nitromethane, dinitromethane, and trinitromethane.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: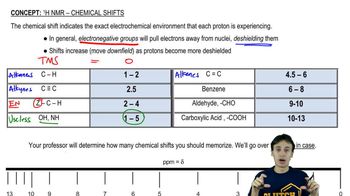



 11:44m
11:44m