Here are the essential concepts you must grasp in order to answer the question correctly.
Lone Pairs
Lone pairs are pairs of valence electrons that are not involved in bonding and are localized on a single atom. They play a crucial role in determining the geometry and reactivity of molecules. Understanding where lone pairs are located helps predict molecular shapes and the potential for hydrogen bonding or other interactions.
Recommended video:
Lewis Structures
Lewis structures are diagrams that represent the bonding between atoms in a molecule and the lone pairs of electrons. They provide a visual way to understand how atoms are connected and the distribution of electrons. Drawing Lewis structures accurately is essential for predicting molecular behavior and reactivity.
Recommended video:
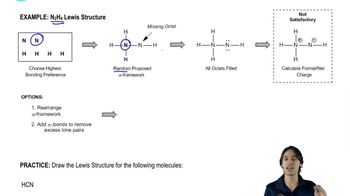
Drawing the Lewis Structure for N2H4.
Resonance Structures
Resonance structures are different ways of drawing the same molecule that show the delocalization of electrons. They are used when a single Lewis structure cannot adequately represent the molecule's electron distribution. Understanding resonance is important for predicting the stability and reactivity of molecules, especially in organic chemistry.
Recommended video:
Drawing Resonance Structures

 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 2:14m
2:14m