Here are the essential concepts you must grasp in order to answer the question correctly.
Nucleophilic Substitution
Nucleophilic substitution is a fundamental reaction mechanism in organic chemistry where a nucleophile attacks an electrophile, resulting in the replacement of a leaving group. In the case of benzonitrile hydrolysis, water acts as the nucleophile, attacking the carbon atom of the nitrile group, leading to the formation of an intermediate that eventually yields benzoate and ammonia.
Recommended video:
Nucleophiles and Electrophiles can react in Substitution Reactions.
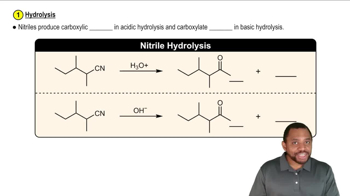
Hydrolysis of Nitriles
Hydrolysis of nitriles involves the reaction of a nitrile with water, typically in the presence of an acid or base, to convert it into a carboxylic acid and ammonia. This process is crucial for understanding the transformation of benzonitrile into benzoate ion and ammonia, as it highlights the role of water in breaking the carbon-nitrogen bond and forming the carboxylate group.
Recommended video:
Review of Nitriles Concept 6
Acid-Base Equilibrium
Acid-base equilibrium is essential in understanding the protonation and deprotonation steps during the hydrolysis reaction. In the case of benzonitrile hydrolysis, the benzoate ion can exist in equilibrium with its protonated form, benzoic acid, depending on the pH of the solution. This concept helps explain the final products and their stability in the reaction environment.
Recommended video:
Determining Acid/Base Equilibrium