Here are the essential concepts you must grasp in order to answer the question correctly.
Cyclic Hemiacetals
Cyclic hemiacetals are formed when an aldehyde or ketone reacts with an alcohol, resulting in a ring structure. This reaction typically occurs in sugars, where the carbonyl group reacts with a hydroxyl group on the same molecule, leading to a stable cyclic form. Understanding the stability and reactivity of these structures is crucial for predicting their formation equilibrium.
Recommended video:
Monosaccharides - Forming Cyclic Hemiacetals
Equilibrium Constant (Kₑq)
The equilibrium constant (Kₑq) quantifies the ratio of the concentration of products to reactants at equilibrium for a given reaction. A higher Kₑq value indicates a greater tendency for the formation of products, in this case, cyclic hemiacetals. Factors influencing Kₑq include sterics, electronic effects, and the stability of the cyclic structure formed.
Recommended video:
The relationship between equilibrium constant and pKa.
Stability of Cyclic Structures
The stability of cyclic structures, such as hemiacetals, is influenced by factors like ring strain, steric hindrance, and the presence of electron-donating or withdrawing groups. More stable cyclic hemiacetals will favor formation, leading to a higher Kₑq. Analyzing the substituents and the overall conformation of the cyclic hemiacetals is essential for predicting which will have the highest equilibrium constant.
Recommended video:
Monosaccharides - Forming Cyclic Hemiacetals
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: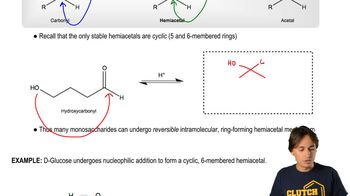



 3:34m
3:34m