Here are the essential concepts you must grasp in order to answer the question correctly.
Free-Radical Bromination
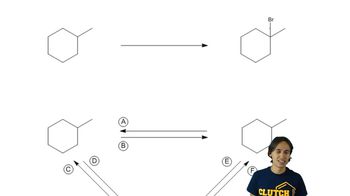
Free-radical bromination is a reaction mechanism where bromine (Br2) reacts with alkanes in the presence of heat or light, leading to the formation of brominated products. This process involves the generation of bromine radicals, which abstract hydrogen atoms from the alkane, resulting in the formation of alkyl radicals that can further react with bromine to yield monobrominated derivatives. Understanding this mechanism is crucial for predicting the products formed from specific alkanes.
Recommended video:
Using the Hammond Postulate to describe radical bromination.
Selectivity in Bromination
Selectivity in bromination refers to the preference of bromine radicals to abstract hydrogen atoms from certain positions in an alkane, leading to different products. This selectivity is influenced by the stability of the resulting alkyl radicals; more stable radicals (like tertiary) are favored over less stable ones (like primary). Recognizing the structure of the alkane helps in predicting which monobrominated derivatives will be formed in good yield.
Recommended video:
Mechanism of Allylic Bromination.
Cycloalkanes vs. Alkanes
Cycloalkanes, such as cyclopentane, differ from linear alkanes in their structure, which can affect the outcomes of reactions like bromination. The ring structure can create steric hindrance and influence the stability of the radicals formed during the reaction. Understanding the differences between cycloalkanes and alkanes is essential for predicting the types of monobrominated derivatives that can be produced from these compounds.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution


 1:08m
1:08m